Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1558: Prevalence, Phenotypical Clinical Clusters and Treatment of Neurobehçet's Disease: Study in Northern Spain
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AH
Alba Herrero-Morant, MD
Hospital Universitario Marqués de Valdecilla
Ontinyent, Spain
Abstract Poster Presenter(s)
Alba Herrero-Morant1, Carmen Alvarez Reguera2, Lara Sánchez-Bilbao2, David Martínez-López2, guillermo Suárez-Amorin2, Raúl fernández-ramón2, José Luis Martín-Varillas3, Cristina Mata4, Miguel Ángel González-Gay5 and Ricardo Blanco6, 1Hospital Universitario Marqués de Valdecilla, Ontinyent, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Hospital de Laredo, Laredo, Cantabria, Spain, 4Hospital Laredo, Santander, Spain, 5Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 6Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Behçet's disease (BD) may present with different clinical phenotypes. Ocular and Neurobehçet's Disease (NBD) are severe complications. Data on NBD epidemiology, clinical phenotype and therapy are scarce and controversial. In a wide unselected single-center series of BD our aims were to assess a) NBD prevalence, b) associations with other clinical clusters and c) treatment.
Methods: Cross-sectional study of all 120 patients diagnosed with BD in Northern Spain, between January 1, 1999 to December 31, 2019. Finally, 96 patients were included in this study according to 2014 International Criteria for Behçet Disease (ICBD). NBD was diagnosed according to the International Consensus Recommendation (ICR) criteria.
Results: NBD was diagnosed in 23 of 96 (24%) patients (15 women/8 men) (mean age: 44±13.9 years). NBD was classified as parenchymatous (n=10, 43.5%), non-parenchymatous (n=10, 43.5%) and mixed (n=3, 13%). HLAB51 was positive in 5 out of 13 (38.4%) patients tested. The main cluster of clinical associations were oral aphthae (n=20, 87%); ocular (n=14, 60.9%), cutaneous (n=10, 43.5%), articular (n=9, 39.1%), vascular (n=4, 17.4%) and intestinal (n=1, 8.7%) involvement (FIGURE). Treatments were oral corticosteroids (n=16; 69.6%; mean maximum dose 42±12.5 mg/ day, conventional immunosuppressants (n=13, 56.5%) and Biological Therapy (BT) (n= 7; 30.4%). BT was used in patients who were refractory to conventional immunosuppressants. Monoclonal anti-TNFα were used as the first option in all patients who received BT. In 3 out of 7 (42.7%) patients BT was switched due to inefficacy. TABLE shows the main NBD clinical subtypes and treatment. Complete remission was achieved in 18 of 23 cases (78.2%), partial response in 2 out of 23 cases (8.7%). No severe adverse effects were observed.
Conclusion: NBD was observed in 24% of patients with BD. The most frequent clinical clusters of NBD were oral aphthae and ocular involvement. All patients treated with either conventional immunosuppressant or BT achieved clinical remission.
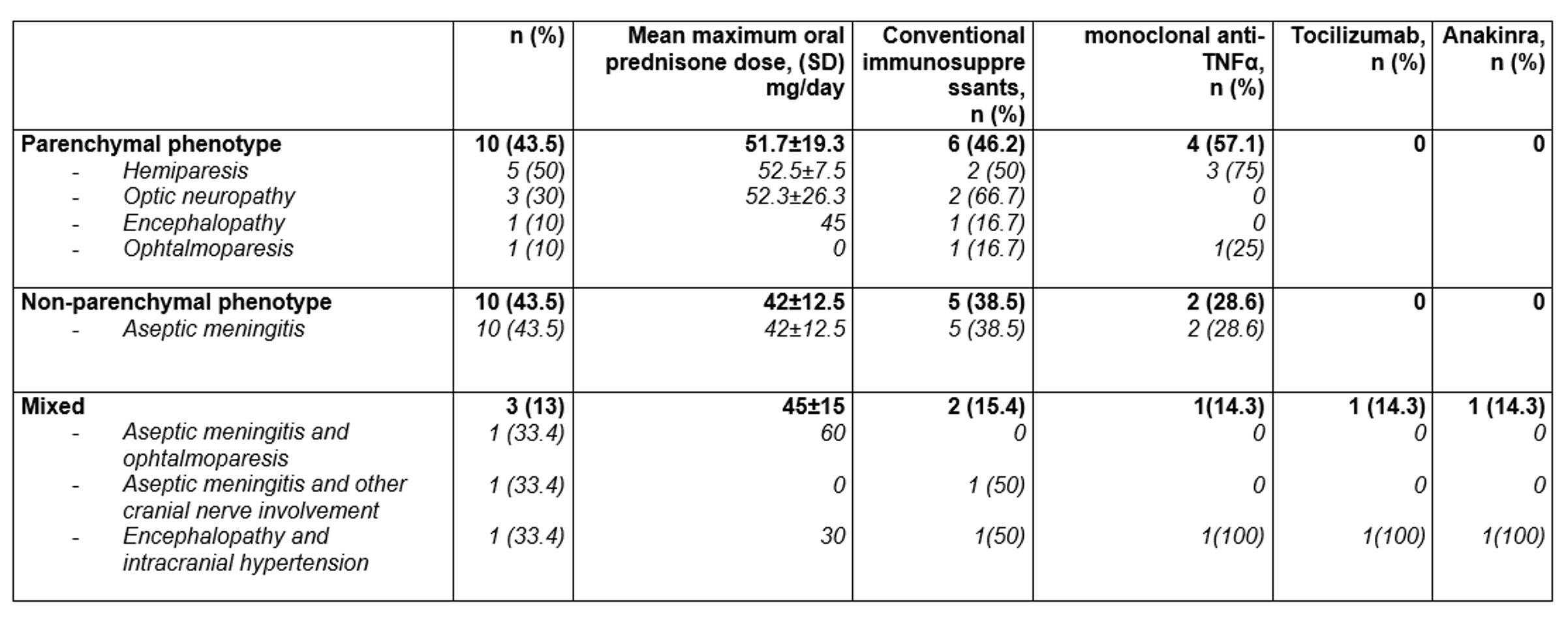 TABLE: Main clinical features and treatment of 23 patients with Neurobehçet’s disease
TABLE: Main clinical features and treatment of 23 patients with Neurobehçet’s disease
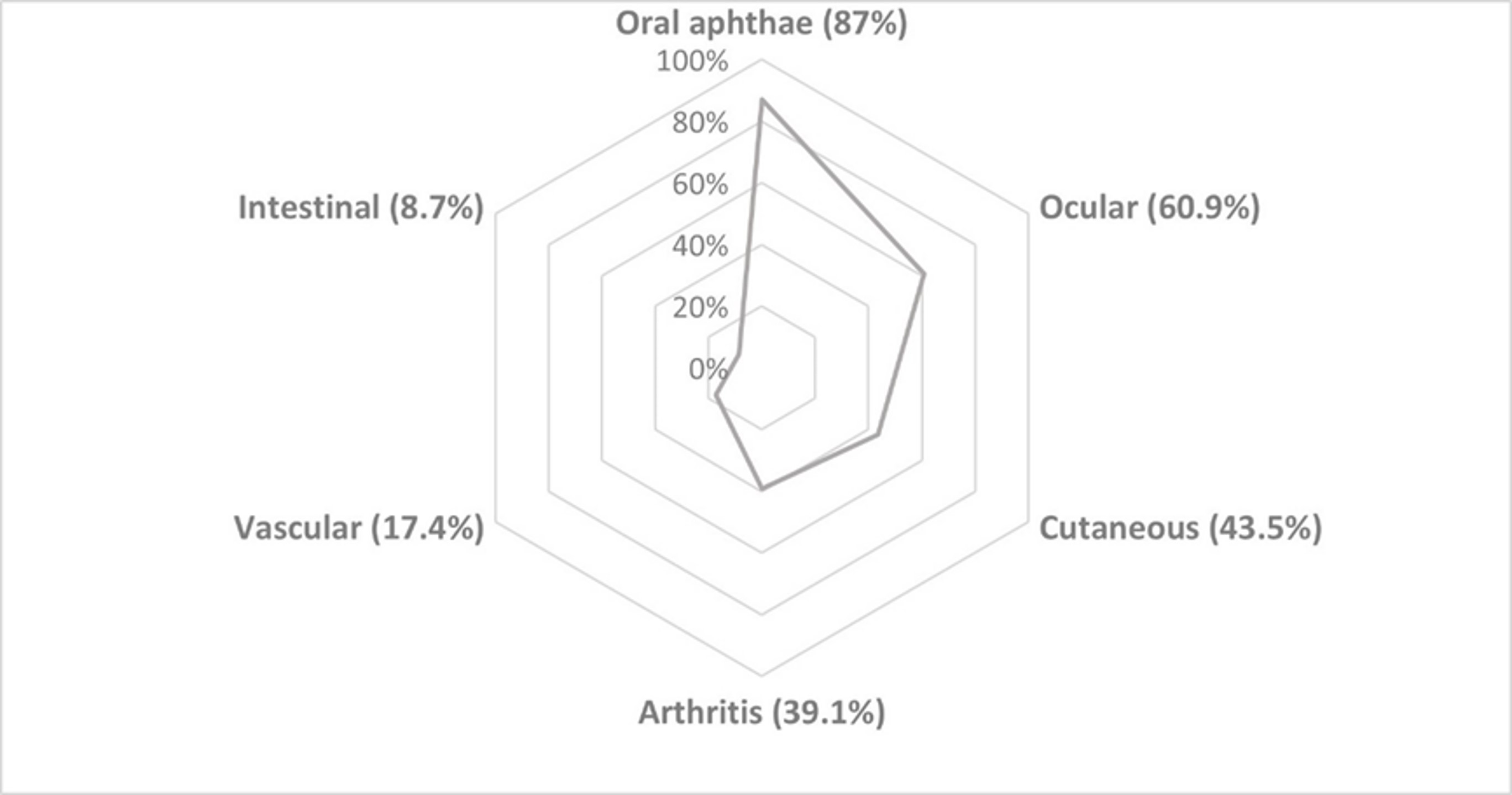 FIGURE: Clusters of Neurobehçet’s clinical associations
FIGURE: Clusters of Neurobehçet’s clinical associations
Disclosures: A. Herrero-Morant, None; C. Alvarez Reguera, None; L. Sánchez-Bilbao, Eli Lilly; D. Martínez-López, None; g. Suárez-Amorin, None; R. fernández-ramón, None; J. Martín-Varillas, AbbVie/Abbott, Pfizer, Janssen, UCB, Celgene; C. Mata, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Behçet's disease (BD) may present with different clinical phenotypes. Ocular and Neurobehçet's Disease (NBD) are severe complications. Data on NBD epidemiology, clinical phenotype and therapy are scarce and controversial. In a wide unselected single-center series of BD our aims were to assess a) NBD prevalence, b) associations with other clinical clusters and c) treatment.
Methods: Cross-sectional study of all 120 patients diagnosed with BD in Northern Spain, between January 1, 1999 to December 31, 2019. Finally, 96 patients were included in this study according to 2014 International Criteria for Behçet Disease (ICBD). NBD was diagnosed according to the International Consensus Recommendation (ICR) criteria.
Results: NBD was diagnosed in 23 of 96 (24%) patients (15 women/8 men) (mean age: 44±13.9 years). NBD was classified as parenchymatous (n=10, 43.5%), non-parenchymatous (n=10, 43.5%) and mixed (n=3, 13%). HLAB51 was positive in 5 out of 13 (38.4%) patients tested. The main cluster of clinical associations were oral aphthae (n=20, 87%); ocular (n=14, 60.9%), cutaneous (n=10, 43.5%), articular (n=9, 39.1%), vascular (n=4, 17.4%) and intestinal (n=1, 8.7%) involvement (FIGURE). Treatments were oral corticosteroids (n=16; 69.6%; mean maximum dose 42±12.5 mg/ day, conventional immunosuppressants (n=13, 56.5%) and Biological Therapy (BT) (n= 7; 30.4%). BT was used in patients who were refractory to conventional immunosuppressants. Monoclonal anti-TNFα were used as the first option in all patients who received BT. In 3 out of 7 (42.7%) patients BT was switched due to inefficacy. TABLE shows the main NBD clinical subtypes and treatment. Complete remission was achieved in 18 of 23 cases (78.2%), partial response in 2 out of 23 cases (8.7%). No severe adverse effects were observed.
Conclusion: NBD was observed in 24% of patients with BD. The most frequent clinical clusters of NBD were oral aphthae and ocular involvement. All patients treated with either conventional immunosuppressant or BT achieved clinical remission.
 TABLE: Main clinical features and treatment of 23 patients with Neurobehçet’s disease
TABLE: Main clinical features and treatment of 23 patients with Neurobehçet’s disease FIGURE: Clusters of Neurobehçet’s clinical associations
FIGURE: Clusters of Neurobehçet’s clinical associationsDisclosures: A. Herrero-Morant, None; C. Alvarez Reguera, None; L. Sánchez-Bilbao, Eli Lilly; D. Martínez-López, None; g. Suárez-Amorin, None; R. fernández-ramón, None; J. Martín-Varillas, AbbVie/Abbott, Pfizer, Janssen, UCB, Celgene; C. Mata, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

