Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1536: Progressive Interstitial Lung Disease Is Frequent Also in Late Disease Stages in Systemic Sclerosis Patients from EUSTAR
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AH
Anna-Maria Hoffmann-Vold, MD, PhD
Oslo University Hospital
Oslo, Norway
Abstract Poster Presenter(s)
Anna-Maria Hoffmann-Vold1, Cathrine Brunborg1, Paolo Airò2, Lidia P. Ananyeva3, László Czirják4, Serena Guiducci5, Eric Hachulla6, MENGTAO LI7, Carina Mihai8, Gabriela Riemekasten9, Petros P. Sfikakis10, Gabriele Valentini11, Otylia Kowal-Bielecka12, Yannick Allanore13 and Oliver Distler8, 1Oslo University Hospital, Oslo, Norway, 2Rheumatology and Clinical Immunology Unit, ASST Spedali Civili, Brescia, Italy, 3V.A. Nasonova Research Institute of Rheumatology, Moscow, Russia, 4Medical school of Pécs, Pecs, Hungary, 5University of Firenze, Firenze, Italy, 6University of Lille, Lille, France, 7Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, National Clinical Research Center for Dermatologic and Immunologic Diseases, Ministry of Science & Technology, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, State Key Laboratory of Complex Severe and Rare Diseases, Ministry of Science & Technology, Beijing, China, 8Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 9University Clinic Schleswit-Holstein (UKSH), Luebeck, Germany, 10Joint Academic Rheumatology Program, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece, 11Università della Campania “Luigi Vanvitelli”, Napoli, Italy, 12Medical University of Bialystok, Bialystok, Poland, 13Department of Rheumatology A, Descartes University, APHP, Cochin Hospital, Paris, France, Paris, France
Background/Purpose: Short disease duration is a well known predictor for progressive systemic sclerosis-associated interstitial lung disease (SSc-ILD), but studies assessing ILD progression in later disease stages are lacking. To individually tailor management of ILD in SSc patients in clinical practice it is, however, of high importance to understand disease behaviour also in patients with late stage disease. The objective was to analyse ILD progression in SSc-ILD patients from the EUSTAR cohort segregated by subgroups of disease duration.
Methods: We segregated SSc-ILD patients into four categories of disease duration (≤3 years, >3-≤7 years, >7-≤15 years and >15 years after onset of Raynaud's phenomenon). We assessed progressive ILD, defined as absolute forced vital capacity (FVC) decline >10% or FVC decline ≥10% and FVC decline 5–10% and diffusing capacity of the lungs for carbon monoxide (DLCO) decline ≥15% (composite decline) over the first and second 12+/-3 months period after first registration (baseline) into EUSTAR. Clinical characteristics, pulmonary involvement, treatment at first registration and ILD progression were evaluated by descriptive statistics.
Results: In total, 2258 SSc-ILD patients were included, with 469 (20.8%) having a disease duration ≤3 years, 550 (24.4%) between >3-≤7 years, 752 (33.3%) between >7-≤15 years and 488 (21.6%) of >15 years (Table). Baseline characteristics and treatment patterns differed between the four subgroups, with more younger male patients with diffuse cutaneous SSc, anti-topoisomerase I antibody and higher Rodnan skin score having ≤3 years disease duration (Figure 1). Lung function with FVC and DLCO were similar between the four groups (Table). Notably, in the first and second 12+/-3 months periods after first registration in the EUSTAR database, there were no significant difference in FVC decline >10% or composite FVC and DLCO decline within the four subgroups. For example, patients with disease duration >7-≤15 years and >15 years frequently showed disease progression of FVC >10%: 41/347 (11.8%) and 32/228 (14%) compared to 38/244 (15.6%) and 33/273 (15.6%) for disease duration ≤3 years and >3-≤7 years (P=0.529), respectively (Figure 2).
Conclusion: It was long believed that ILD burned out in late disease stages. In our analysis of ILD progression by four disease duration categories, we showed that ILD frequently progressed also in late disease stages. This has important implications for clinical practise, as SSc patients need to be regularly monitored for ILD progression independent of disease duration.
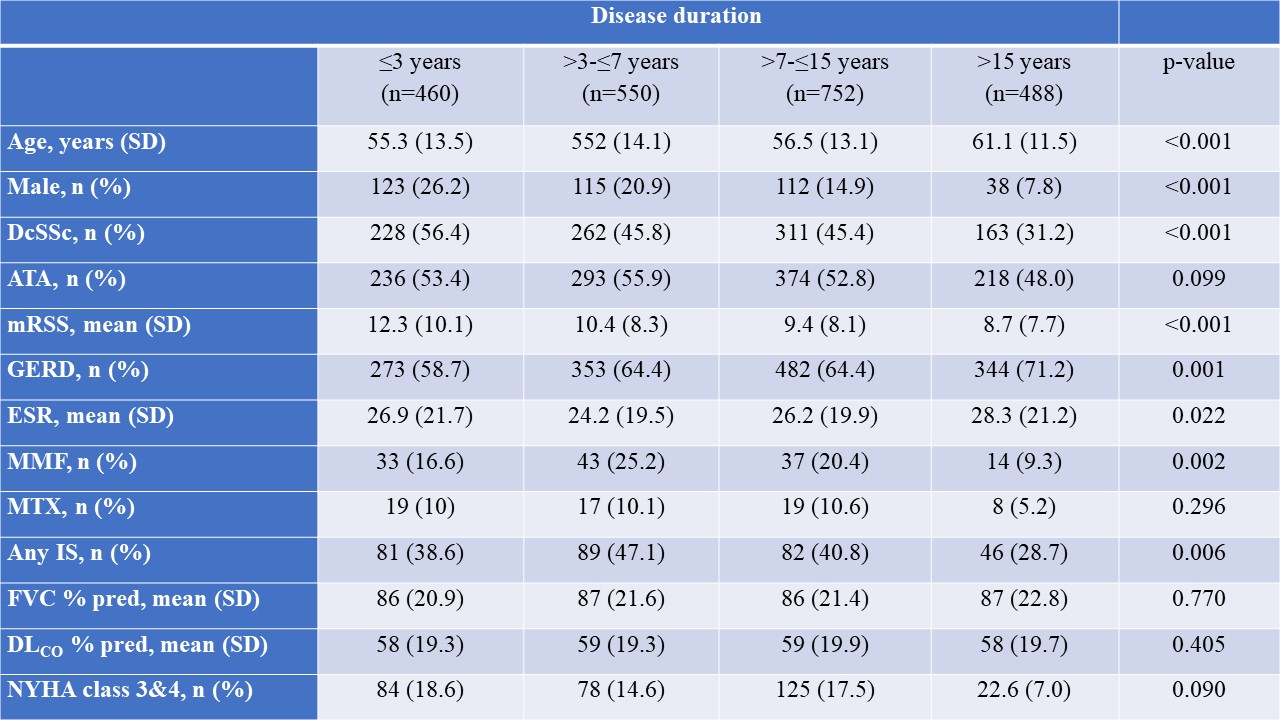 Table. Demographics and baseline clinical characteristics of EUSTAR patients
Table. Demographics and baseline clinical characteristics of EUSTAR patients
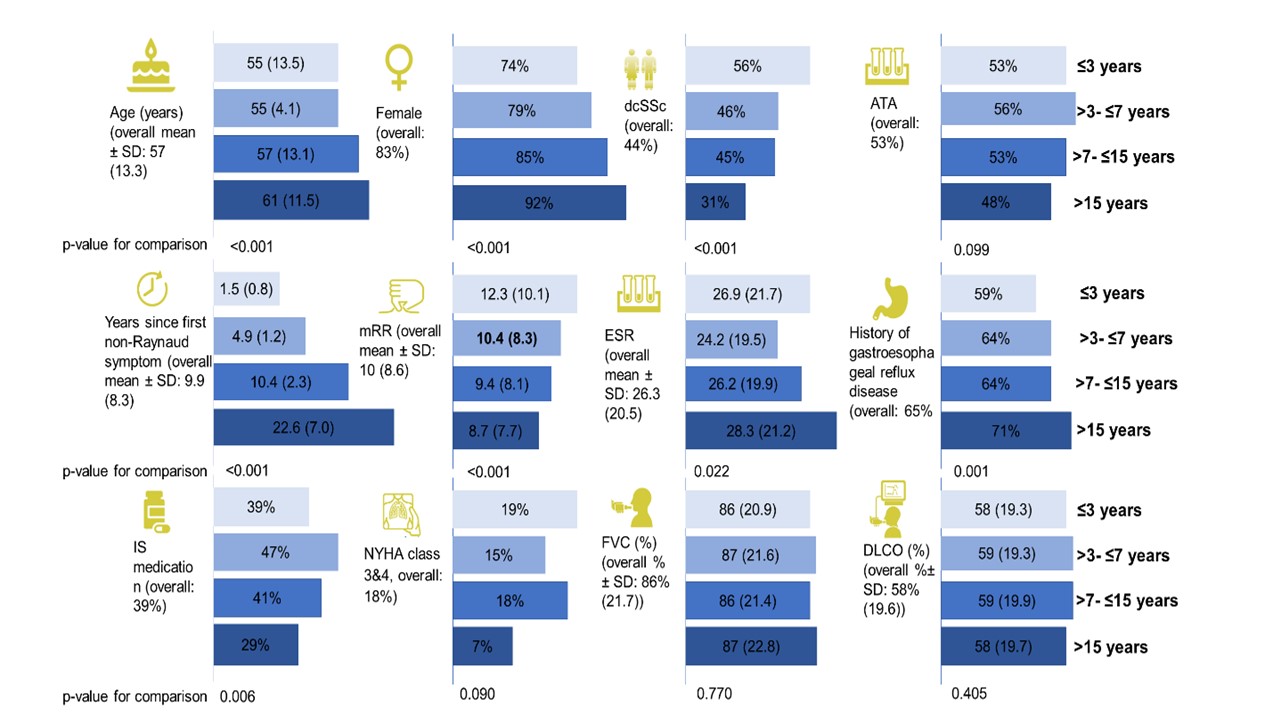 Figure 1: Factors associated with ILD progression segregated by disease duration
Figure 1: Factors associated with ILD progression segregated by disease duration
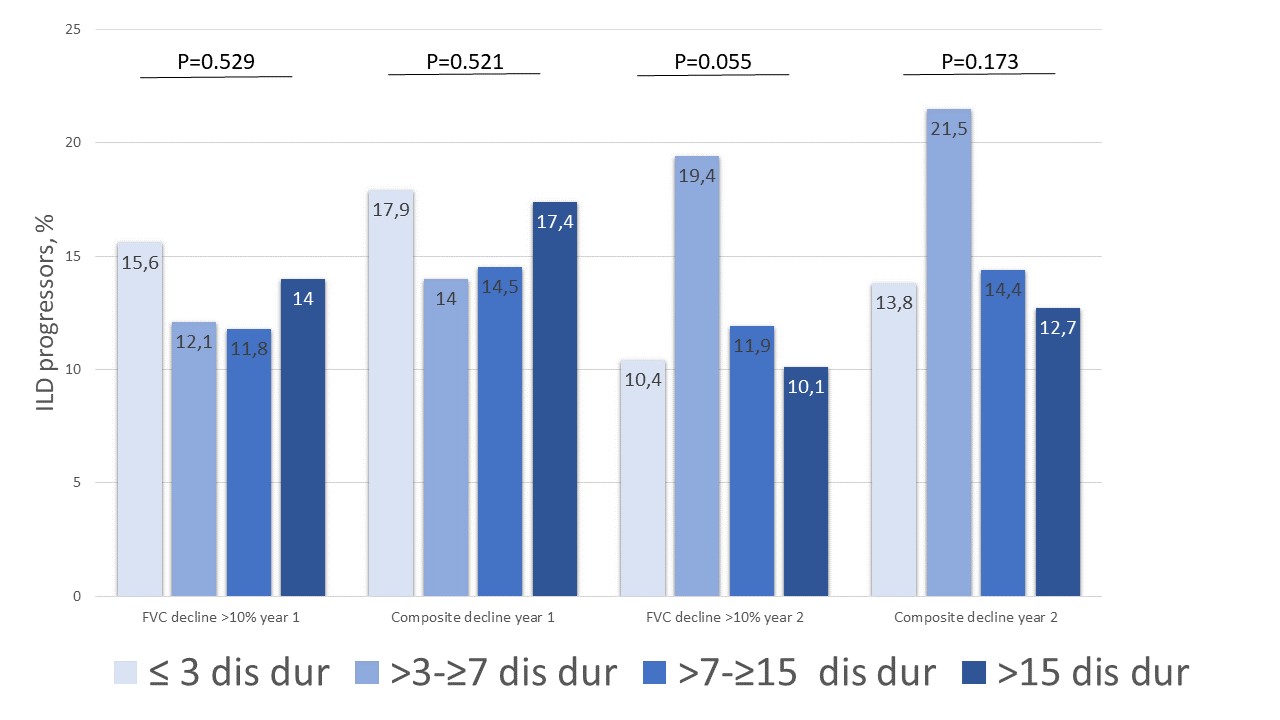 Figure 2: Number of ILD progressors segregated by disease duration
Figure 2: Number of ILD progressors segregated by disease duration
Disclosures: A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche; C. Brunborg, None; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; L. Ananyeva, Boehringer-Ingelheim; L. Czirják, Boehringer Ingelheim, Actelion, MSD, Novartis, Pfizer; S. Guiducci, None; E. Hachulla, GSK, Roche-Chugai, Johnson & Johnson, Boehringer Ingelheim, CSL Behring, Sanofi Genzyme; M. LI, None; C. Mihai, Boehringer-Ingelheim, Mepha, MEDTalks, Roche, Janssen; G. Riemekasten, Boehringer Ingelheim; P. Sfikakis, Pfizer, AbbVie/Abbott, Novartis, Amgen, Janssen, Boehringer-Ingelheim, Celgene, Eli Lilly; G. Valentini, Boehringer Ingelheim, Sanofi/BMS; O. Kowal-Bielecka, CSL Behring, Boehringer Ingelheim, Gilead, Novartis, Abbvie, Janssen-Cilag, Medac, MSD, Roche; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation.
Background/Purpose: Short disease duration is a well known predictor for progressive systemic sclerosis-associated interstitial lung disease (SSc-ILD), but studies assessing ILD progression in later disease stages are lacking. To individually tailor management of ILD in SSc patients in clinical practice it is, however, of high importance to understand disease behaviour also in patients with late stage disease. The objective was to analyse ILD progression in SSc-ILD patients from the EUSTAR cohort segregated by subgroups of disease duration.
Methods: We segregated SSc-ILD patients into four categories of disease duration (≤3 years, >3-≤7 years, >7-≤15 years and >15 years after onset of Raynaud's phenomenon). We assessed progressive ILD, defined as absolute forced vital capacity (FVC) decline >10% or FVC decline ≥10% and FVC decline 5–10% and diffusing capacity of the lungs for carbon monoxide (DLCO) decline ≥15% (composite decline) over the first and second 12+/-3 months period after first registration (baseline) into EUSTAR. Clinical characteristics, pulmonary involvement, treatment at first registration and ILD progression were evaluated by descriptive statistics.
Results: In total, 2258 SSc-ILD patients were included, with 469 (20.8%) having a disease duration ≤3 years, 550 (24.4%) between >3-≤7 years, 752 (33.3%) between >7-≤15 years and 488 (21.6%) of >15 years (Table). Baseline characteristics and treatment patterns differed between the four subgroups, with more younger male patients with diffuse cutaneous SSc, anti-topoisomerase I antibody and higher Rodnan skin score having ≤3 years disease duration (Figure 1). Lung function with FVC and DLCO were similar between the four groups (Table). Notably, in the first and second 12+/-3 months periods after first registration in the EUSTAR database, there were no significant difference in FVC decline >10% or composite FVC and DLCO decline within the four subgroups. For example, patients with disease duration >7-≤15 years and >15 years frequently showed disease progression of FVC >10%: 41/347 (11.8%) and 32/228 (14%) compared to 38/244 (15.6%) and 33/273 (15.6%) for disease duration ≤3 years and >3-≤7 years (P=0.529), respectively (Figure 2).
Conclusion: It was long believed that ILD burned out in late disease stages. In our analysis of ILD progression by four disease duration categories, we showed that ILD frequently progressed also in late disease stages. This has important implications for clinical practise, as SSc patients need to be regularly monitored for ILD progression independent of disease duration.
 Table. Demographics and baseline clinical characteristics of EUSTAR patients
Table. Demographics and baseline clinical characteristics of EUSTAR patients Figure 1: Factors associated with ILD progression segregated by disease duration
Figure 1: Factors associated with ILD progression segregated by disease duration Figure 2: Number of ILD progressors segregated by disease duration
Figure 2: Number of ILD progressors segregated by disease durationDisclosures: A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche; C. Brunborg, None; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; L. Ananyeva, Boehringer-Ingelheim; L. Czirják, Boehringer Ingelheim, Actelion, MSD, Novartis, Pfizer; S. Guiducci, None; E. Hachulla, GSK, Roche-Chugai, Johnson & Johnson, Boehringer Ingelheim, CSL Behring, Sanofi Genzyme; M. LI, None; C. Mihai, Boehringer-Ingelheim, Mepha, MEDTalks, Roche, Janssen; G. Riemekasten, Boehringer Ingelheim; P. Sfikakis, Pfizer, AbbVie/Abbott, Novartis, Amgen, Janssen, Boehringer-Ingelheim, Celgene, Eli Lilly; G. Valentini, Boehringer Ingelheim, Sanofi/BMS; O. Kowal-Bielecka, CSL Behring, Boehringer Ingelheim, Gilead, Novartis, Abbvie, Janssen-Cilag, Medac, MSD, Roche; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation.

