Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0331: Anti-dsDNA Antibodies by Multiplex Flow Immunoassay and Critihidia Luciliae Assays in NYU Lupus Registry: Discordance, Association with Nephritis, and Disease Flare Predictive Value
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- DZ
Devyn Zaminski, BA
NYU School of Medicine
New York, NY, United States
Abstract Poster Presenter(s)
Devyn Zaminski1, Amit Saxena1, Peter Izmirly2, Jill Buyon2 and H Michael Belmont1, 1NYU School of Medicine, New York, NY, 2NYU Grossman School of Medicine, New York, NY
Background/Purpose: SLE is characterized by autoantibody production. The most common lupus-specific serology is the anti-dsDNA antibody (anti-DNA). Traditionally, anti-DNA is measured by an enzyme immunoassay or equivalent such as multiplex flow immunoassay (EIA), which is considered sensitive. In contrast, the crithidia luciliae immunofluorescence test (CLIFT) is considered more specific. Serial measurement of anti-DNA is often used to monitor lupus disease activity. With NYU's extensive multi-race/ethnic lupus registry, we studied the relationship between these two methods, their association with lupus nephritis (LN), and their ability to predict subsequent flares.
Methods: Using the NYU Lupus Registry of patients who meet ACR, SLICC, or EULAR criteria, we identified patients who had one or more simultaneous anti-DNA results by multiplex EIA and CLIFT. We report on their concordance (e.g., always, never, or fluctuating), association with LN, and ability to predict flare within 90 days using the SELENA-SLEDAI Flare Index. To account for degree of positivity, we defined tertiles for EIA and CLIFT as low positive [11-50 and 1:10-1:40], mild positive [51-200 and 1:80-1:320], and high positive [ > 200 and > 1:640].
Results: 207 patients had one or more paired anti-DNA results generating 586 paired results (Table 1). Cohort demographics: 92% Female, 22% Hispanic ethnicity, 24% Black, 16% Asian, 49% White, 10% Other. Overall, 377 pairs were always concordant, and 209 were never concordant. 236 pairs demonstrated titer concordance and 350 with titer discordance. Of the 207 patients, 64 patients had only one and 143 patients had two or more paired tests. Of the 64 patients, 46 were always concordant: 18 had positive EIA/CLIFT and 28 had negative EIA/CLIFT. The remaining 18 patients were never concordant: 8 had +EIA/-CLIFT and 10 had –EIA/+CLIFT. The concordance of the 143 patients with multiple paired results: 73 always, 23 never, and 47 fluctuating. Whether by one or multiple paired tests, 41/207 patients were never concordant. 100 of the 207 patients had LN associated with +EIA in 60 and +CLIFT in 72 (Table 2). Hypocomplementemia was present in 88% of +EIA and 89% of +CLIFT patients with LN. 51 visits in 42 patients had paired anti-DNA results and a SELENA-SLEDAI Flare Index assessment within 90 days. 7 patients had mild flares with +EIA in 4 and +CLIFT in 3. 4 patients had severe flares with +EIA and +CLIFT in 3 (Table 3). Low C3 and or C4 occurred in 1 of 7 (14%) mild flares and in 4 of 4 (100%) severe flares.
Conclusion: Our data demonstrate that discordance of positivity between two assays for anti-DNA occurred in 41/207 (20%) patients, in 207/586 (36%) visits and in 350/586 (60%) visits magnitude of positivity nonconcordant. EIA positivity is associated with LN less often than CLIFT positivity. Flares were infrequent and associated with either EIA or CLIFT positivity, with severe flares more likely if accompanied by hypocomplementemia. We recommend the utility of more than one anti-DNA assay in routine monitoring for lupus disease activity.
.jpg) Table 1 586 total visits with paired EIA and CLIFT. H = high positive M = mild positive L = low positive defined in Methods section
Table 1 586 total visits with paired EIA and CLIFT. H = high positive M = mild positive L = low positive defined in Methods section
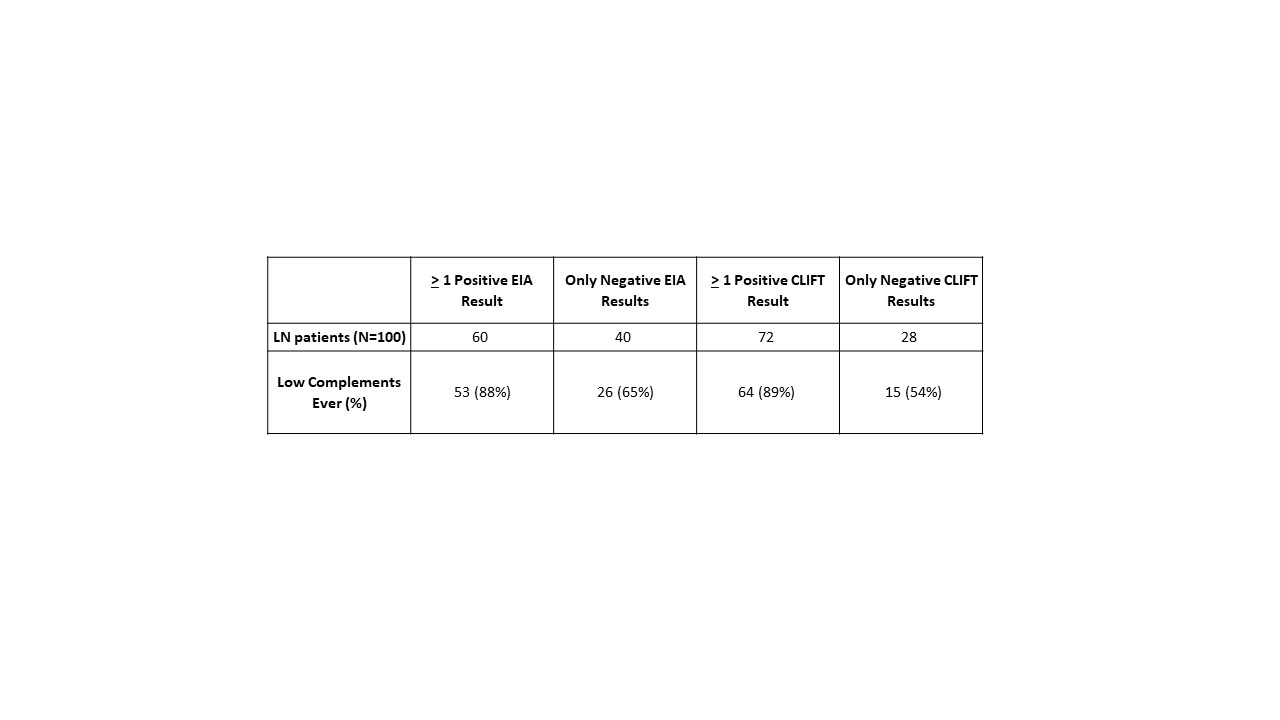 Table 2 Relationship between EIA, CLIFT and hypocomplementemia with lupus nephritis. Chi-square test comparing significance between 60/100 v 72/100 (EIA/CLIFT) p = 0.07
Table 2 Relationship between EIA, CLIFT and hypocomplementemia with lupus nephritis. Chi-square test comparing significance between 60/100 v 72/100 (EIA/CLIFT) p = 0.07
.jpg) Table 3 Relationship between EIA, CLIFT, hypocomplementemia and flare within 90 days
Table 3 Relationship between EIA, CLIFT, hypocomplementemia and flare within 90 days
Disclosures: D. Zaminski, None; A. Saxena, Eli Lilly, AstraZeneca, GlaxoSmithKlein(GSK), Kezar Life Sciences, Bristol-Myers Squibb(BMS); P. Izmirly, Momenta/Janssen; J. Buyon, None; H. Belmont, None.
Background/Purpose: SLE is characterized by autoantibody production. The most common lupus-specific serology is the anti-dsDNA antibody (anti-DNA). Traditionally, anti-DNA is measured by an enzyme immunoassay or equivalent such as multiplex flow immunoassay (EIA), which is considered sensitive. In contrast, the crithidia luciliae immunofluorescence test (CLIFT) is considered more specific. Serial measurement of anti-DNA is often used to monitor lupus disease activity. With NYU's extensive multi-race/ethnic lupus registry, we studied the relationship between these two methods, their association with lupus nephritis (LN), and their ability to predict subsequent flares.
Methods: Using the NYU Lupus Registry of patients who meet ACR, SLICC, or EULAR criteria, we identified patients who had one or more simultaneous anti-DNA results by multiplex EIA and CLIFT. We report on their concordance (e.g., always, never, or fluctuating), association with LN, and ability to predict flare within 90 days using the SELENA-SLEDAI Flare Index. To account for degree of positivity, we defined tertiles for EIA and CLIFT as low positive [11-50 and 1:10-1:40], mild positive [51-200 and 1:80-1:320], and high positive [ > 200 and > 1:640].
Results: 207 patients had one or more paired anti-DNA results generating 586 paired results (Table 1). Cohort demographics: 92% Female, 22% Hispanic ethnicity, 24% Black, 16% Asian, 49% White, 10% Other. Overall, 377 pairs were always concordant, and 209 were never concordant. 236 pairs demonstrated titer concordance and 350 with titer discordance. Of the 207 patients, 64 patients had only one and 143 patients had two or more paired tests. Of the 64 patients, 46 were always concordant: 18 had positive EIA/CLIFT and 28 had negative EIA/CLIFT. The remaining 18 patients were never concordant: 8 had +EIA/-CLIFT and 10 had –EIA/+CLIFT. The concordance of the 143 patients with multiple paired results: 73 always, 23 never, and 47 fluctuating. Whether by one or multiple paired tests, 41/207 patients were never concordant. 100 of the 207 patients had LN associated with +EIA in 60 and +CLIFT in 72 (Table 2). Hypocomplementemia was present in 88% of +EIA and 89% of +CLIFT patients with LN. 51 visits in 42 patients had paired anti-DNA results and a SELENA-SLEDAI Flare Index assessment within 90 days. 7 patients had mild flares with +EIA in 4 and +CLIFT in 3. 4 patients had severe flares with +EIA and +CLIFT in 3 (Table 3). Low C3 and or C4 occurred in 1 of 7 (14%) mild flares and in 4 of 4 (100%) severe flares.
Conclusion: Our data demonstrate that discordance of positivity between two assays for anti-DNA occurred in 41/207 (20%) patients, in 207/586 (36%) visits and in 350/586 (60%) visits magnitude of positivity nonconcordant. EIA positivity is associated with LN less often than CLIFT positivity. Flares were infrequent and associated with either EIA or CLIFT positivity, with severe flares more likely if accompanied by hypocomplementemia. We recommend the utility of more than one anti-DNA assay in routine monitoring for lupus disease activity.
.jpg) Table 1 586 total visits with paired EIA and CLIFT. H = high positive M = mild positive L = low positive defined in Methods section
Table 1 586 total visits with paired EIA and CLIFT. H = high positive M = mild positive L = low positive defined in Methods section Table 2 Relationship between EIA, CLIFT and hypocomplementemia with lupus nephritis. Chi-square test comparing significance between 60/100 v 72/100 (EIA/CLIFT) p = 0.07
Table 2 Relationship between EIA, CLIFT and hypocomplementemia with lupus nephritis. Chi-square test comparing significance between 60/100 v 72/100 (EIA/CLIFT) p = 0.07.jpg) Table 3 Relationship between EIA, CLIFT, hypocomplementemia and flare within 90 days
Table 3 Relationship between EIA, CLIFT, hypocomplementemia and flare within 90 daysDisclosures: D. Zaminski, None; A. Saxena, Eli Lilly, AstraZeneca, GlaxoSmithKlein(GSK), Kezar Life Sciences, Bristol-Myers Squibb(BMS); P. Izmirly, Momenta/Janssen; J. Buyon, None; H. Belmont, None.

