Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0372–0402) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster I
0399: ASAS Recommendations for Requesting and Reporting Imaging in Patients with Suspected Axial Spondyloarthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- TD
Torsten Diekhoff, MD
Charité Universitätsmedizin Berlin
Berlin, Germany
Abstract Poster Presenter(s)
Torsten Diekhoff1, Iris Eshed2, Chiara Giraudo3, Hildrun Haibel4, Kay-Geert Hermann5, Manouk de Hooge6, Lennart Jans7, Anne Jurik8, Robert G Lambert9, Pedro Machado10, Walter P Maksymowych11, Michael Mallinson12, Helena Marzo-Ortega13, Victoria Navarro-Compán14, Susanne Pedersen15, Mikkel Østergaard16, Monique Reijnierse17, Martin Rudwaleit18, Fernando Andres Sommerfleck19, Ulrich Weber20, Xenofon Baraliakos21 and Denis Poddubnyy22, 1Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Sheba Medical Center, Tel Aviv, Israel, 3University of Padova, Padova, Italy, 4Charité - Universitätsmedizin, Berlin, Berlin, Germany, 5Charité - Universitätsmedizin Berlin, Berlin, Germany, 6Ghent University Hospital, Luxembourg, Luxembourg, 7Ghent University Hospital, Ghent, Belgium, 8Aarhus University Hospital, Aarhus, Denmark, 9University of Alberta, Edmonton, AB, Canada, 10University College London, London, United Kingdom, 11Department of Medicine, University of Alberta, Edmonton, AB, Canada, 12ASIF Axial Spondyloarthritis International Federation, London, United Kingdom, 13Leeds Teaching Hospitals Trust and University of Leeds, Leeds, United Kingdom, 14Department of Rheumatology, La Paz University Hospital, IdiPaz, Madrid, Spain, 15Rigshospitalet, København, Denmark, 16Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 17Leiden University Medical Center, Leiden, Netherlands, 18University of Bielefeld, Klinikum Bielefeld, Bielefeld; Germany Klinikum Bielefeld and Charité Berlin, Germany, and Gent University, Gent, Belgium, 19Sanatorio Julio Mendez, Buenos Aires, Argentina, 20Practice Buchsbaum Schaffhausen, Schaffhausen, Switzerland, 21Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 22Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany
Background/Purpose: Clinicians face uncertainties in their daily practice when requesting imaging for patients with suspicion of axial spondyloarthritis (axSpA) or producing a report because requirements and desired information of the other disciplines is sometimes not completely known or understood. This project aimed to develop easy-to-follow consensus recommendations for the standardized communication around imaging of sacroiliac joints and spine for diagnosis in patients with suspected axSpA or its management in clinical practice.
Methods: A task force was established combining radiologists and rheumatologists from the Assessment of SpondyloArthritis international Society (ASAS), two members of young-ASAS and a patient representative. The task force defined the project's aims and developed a project statement. Then, under the reflection of the literature and work of other groups, two survey rounds were designed, and all ASAS members invited to respond: first, to identify items for further consideration, second, to consider the detail of information to be transferred. Finally, ASAS members discussed the recommendations during the ASAS annual workshop in January 2022 and voted for the endorsement.
Results: The recommendations were endorsed by ASAS with approval from 73% of voting members. The final set of recommendation is presented in Figure 1. Six recommendations deal with imaging requests in patients with axSpA. The first three cover clinical features, patients' symptoms and risk factors. Recommendations 4 involves previous imaging and reports, 5 contraindications to imaging or contrast media. Number 6 is about the suspected diagnosis, the reason for the exam, and clinical differential diagnoses. Eleven recommendations refer to the radiology report. The first point addresses clinical information included in the report. Recommendations 2 to 4 instruct on information about the technical conduct of the exam, the use of contrast media and image quality. Necessary imaging findings to be included in the report are listed in recommendations 5 to 7. Finally, recommendations 8 to 11 combine advice for the conclusion, for suggesting additional imaging or referral to a rheumatology expert if a different physician requested the imaging.
Conclusion: The ASAS recommendations provide guidance for requesting and reporting imaging in axSpA and for standardizing communication between rheumatologists and radiologists to improve diagnosis and patient care.
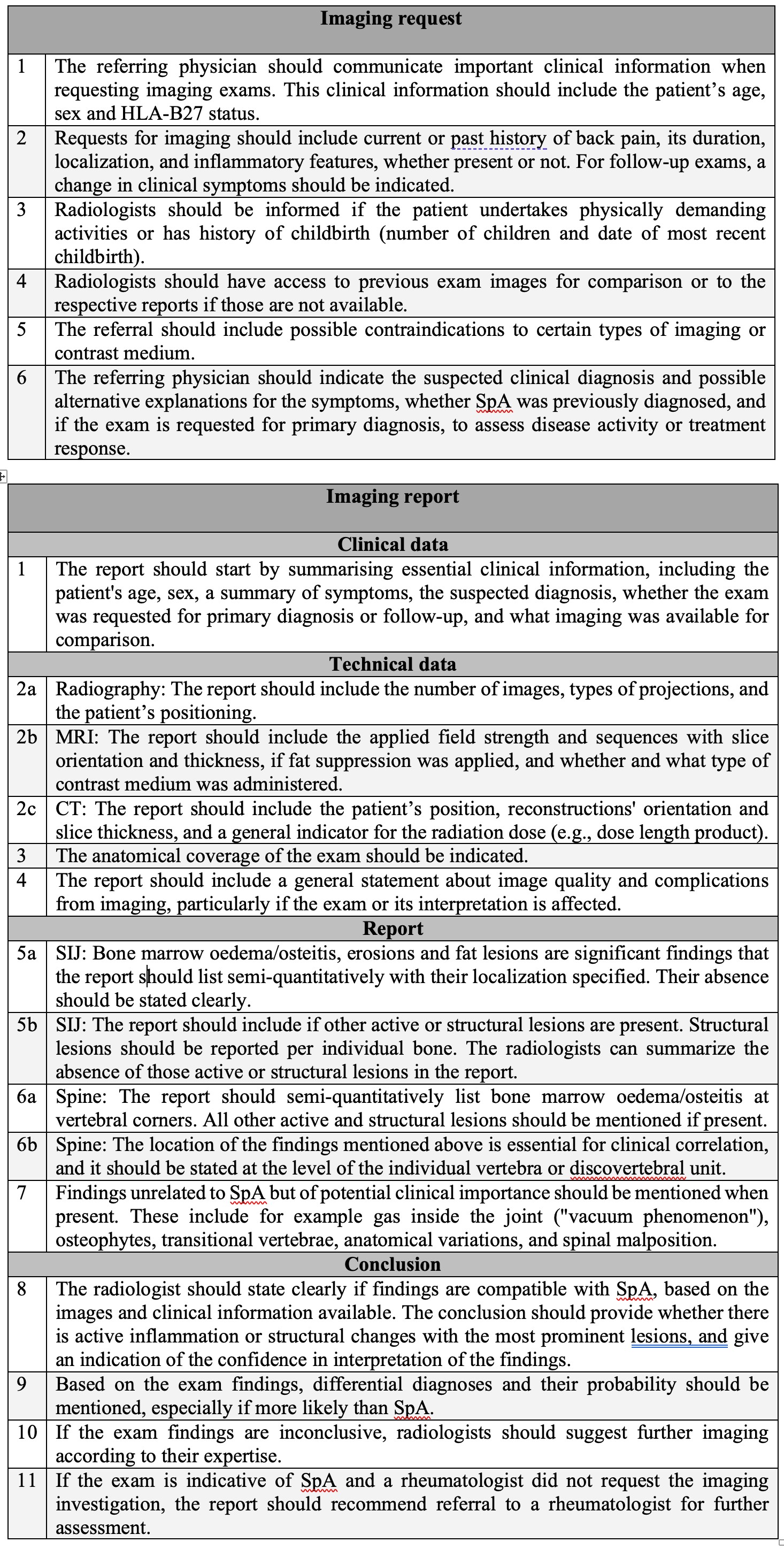 Figure 1: ASAS recommendations for requesting and reporting imaging in patients with suspected axial spondyloarthritis.
Figure 1: ASAS recommendations for requesting and reporting imaging in patients with suspected axial spondyloarthritis.
Disclosures: T. Diekhoff, Novartis, Merck/MSD, Canon MS, Eli Lilly; I. Eshed, None; C. Giraudo, None; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; K. Hermann, AbbVie, Merck/MSD, Pfizer, Novartis, BerlinFlame GmbH; M. de Hooge, None; L. Jans, None; A. Jurik, None; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; M. Mallinson, None; H. Marzo-Ortega, None; V. Navarro-Compán, AbbVie, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, UCB Pharma; S. Pedersen, Novartis, AbbVie/Abbott, UCB, Pfizer, Merck/MSD; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Reijnierse, ASAS, International Skeletal Society; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; F. Sommerfleck, AbbVie/Abbott, Novartis, Janssen; U. Weber, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.
Background/Purpose: Clinicians face uncertainties in their daily practice when requesting imaging for patients with suspicion of axial spondyloarthritis (axSpA) or producing a report because requirements and desired information of the other disciplines is sometimes not completely known or understood. This project aimed to develop easy-to-follow consensus recommendations for the standardized communication around imaging of sacroiliac joints and spine for diagnosis in patients with suspected axSpA or its management in clinical practice.
Methods: A task force was established combining radiologists and rheumatologists from the Assessment of SpondyloArthritis international Society (ASAS), two members of young-ASAS and a patient representative. The task force defined the project's aims and developed a project statement. Then, under the reflection of the literature and work of other groups, two survey rounds were designed, and all ASAS members invited to respond: first, to identify items for further consideration, second, to consider the detail of information to be transferred. Finally, ASAS members discussed the recommendations during the ASAS annual workshop in January 2022 and voted for the endorsement.
Results: The recommendations were endorsed by ASAS with approval from 73% of voting members. The final set of recommendation is presented in Figure 1. Six recommendations deal with imaging requests in patients with axSpA. The first three cover clinical features, patients' symptoms and risk factors. Recommendations 4 involves previous imaging and reports, 5 contraindications to imaging or contrast media. Number 6 is about the suspected diagnosis, the reason for the exam, and clinical differential diagnoses. Eleven recommendations refer to the radiology report. The first point addresses clinical information included in the report. Recommendations 2 to 4 instruct on information about the technical conduct of the exam, the use of contrast media and image quality. Necessary imaging findings to be included in the report are listed in recommendations 5 to 7. Finally, recommendations 8 to 11 combine advice for the conclusion, for suggesting additional imaging or referral to a rheumatology expert if a different physician requested the imaging.
Conclusion: The ASAS recommendations provide guidance for requesting and reporting imaging in axSpA and for standardizing communication between rheumatologists and radiologists to improve diagnosis and patient care.
 Figure 1: ASAS recommendations for requesting and reporting imaging in patients with suspected axial spondyloarthritis.
Figure 1: ASAS recommendations for requesting and reporting imaging in patients with suspected axial spondyloarthritis. Disclosures: T. Diekhoff, Novartis, Merck/MSD, Canon MS, Eli Lilly; I. Eshed, None; C. Giraudo, None; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; K. Hermann, AbbVie, Merck/MSD, Pfizer, Novartis, BerlinFlame GmbH; M. de Hooge, None; L. Jans, None; A. Jurik, None; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; M. Mallinson, None; H. Marzo-Ortega, None; V. Navarro-Compán, AbbVie, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, UCB Pharma; S. Pedersen, Novartis, AbbVie/Abbott, UCB, Pfizer, Merck/MSD; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Reijnierse, ASAS, International Skeletal Society; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; F. Sommerfleck, AbbVie/Abbott, Novartis, Janssen; U. Weber, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.

