Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0330: Assessing the Utility of the Montreal Cognitive Assessment as a Screening Tool for Cognitive Impairment in Patients with SLE Using a Bayesian Item-Response Theory Model
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JD
Juan Pablo Diaz Martinez, PhD
UHN
Toronto, ON, Canada
Abstract Poster Presenter(s)
Juan Pablo Diaz-Martinez1, Michelle Barraclough2, Kimberley Yuen3, Oshrat Tayer-Shifman4, Andrea Knight5, Kathleen Bingham6, Jiandong Su1, Mahta Kakvan1, Carolina Munoz1, Maria Carmela Tartaglia7, Leslet Ruttan8, Joan Wither9, May Choi10, Dennisse Bonilla1, Simone Appenzeller11, Patricia Katz12, Dorcas Beaton13, Robin Green8 and Zahi Touma1, 1Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 2Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Centre for Epidemiology Versus Arthritis, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Toronto, ON, Canada, 3Queen's University School of Medicine, Toronto, ON, Canada, 4Meir Medical Center, Kfar Saba, Israel, 5The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 6Centre for Mental Health, University Health Network; Department of Psychiatry, University of Toronto, Toronto, ON, Canada, 7University of Toronto Krembil Neurosciences Centre, Toronto, ON, Canada, 8University Health Network-Toronto Rehabilitation Institute, Toronto, ON, Canada, 9Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Toronto, ON, Canada, 10Brigham and Women's Hospital | University of Calgary, Calgary, AB, Canada, 11Unicamp, Campinas, São Paulo, Brazil, 12UCSF, San Rafael, CA, 13Institute for Work & Health, Toronto, ON, Canada
Background/Purpose: Cognitive impairment (CI) is one of the most common manifestations of neuropsychiatric syndromes in patients with SLE, with a prevalence ranging from 20% to 80% and a pooled prevalence of 38%. Previous studies have assessed the utility of the Montreal Cognitive Assessment (MoCA) as a screening tool for CI in SLE patients compared to the American College of Rheumatology neuropsychological battery (ACR-NB), where the performance of the MoCA in the identification of CI has been historically evaluated using a total score of 26 as the threshold. Instead of using this threshold, this study fits an item response theory (IRT) model to the MoCA questionnaire and compares its results to the ACR-NB to assess the utility of the MoCA as a stand-alone screening test.
Methods: Adult SLE patients who attended the University of Toronto Lupus Clinic between July 2016 and March 2020 (excluding those with learning disabilities and less than semi-fluent English language skills) were administered the MoCA followed by the ACR-NB. Patients were classified as having CI if they had a z-score of ≤-1.5 in ≥2 domains of the 6 domains of the ACR-NB, and otherwise non-CI. Using a Bayesian framework, different IRT models with a beta-binomial distribution were used in this analysis. IRT models aim to model persons' responses on a set of items measuring one or more latent constructs, which in our case this latent construct corresponds to CI in SLE patients. Specifically, we fitted 1PL and 2PL models, where the 1PL model estimates the "easiness" of each item (i.e., agreement across patients) and the 2PL adds a discrimination parameter for each item (i.e., not assuming that discriminations are to be constant across items). The performance of both IRT models using the MoCA in the identification of CI as classified by the ACR-NB was evaluated using ROC curve analysis and AUC calculation.
Results: A total of 276 SLE patients were enrolled; 88.8% were female and mean age and mean SLE disease duration at study visit were 41.3 ± 12.1 and 14.4 ± 10.1 years, respectively. Based on the ACR-NB, 129 patients (47%) were classified as having CI, 85 (31%) as undetermined CI, and 62 (22%) as non-CI patients. The 1PL model showed that some items (e.g., Orientation) were consistent amongst most individuals (agreement amongst patients) and thus had strongly positive easiness parameters (Figure 1), while other items (e.g., Delayed Recall) were mostly rejected and thus had a low easiness parameter. For the 2PL model, the easiness parameters displayed on the left-hand side of (Figure 2) still showed a similar pattern as in the 1PL, although their estimates were more spread out because of the discrimination estimates being less than 1. CI was poorly predicted by both IRT models (Figure 3) based on MoCA with an area under curve (AUC) of 67.2% for the 1PL model and 66.6% by the 2PL model.
Conclusion: This study found that the utility of the MoCA in screening for CI in SLE patients is limited. Future research should focus on other IRT models with different response distributions in order to determine if the accuracy of the MoCA could be improved by deriving a different weight score for each domain.
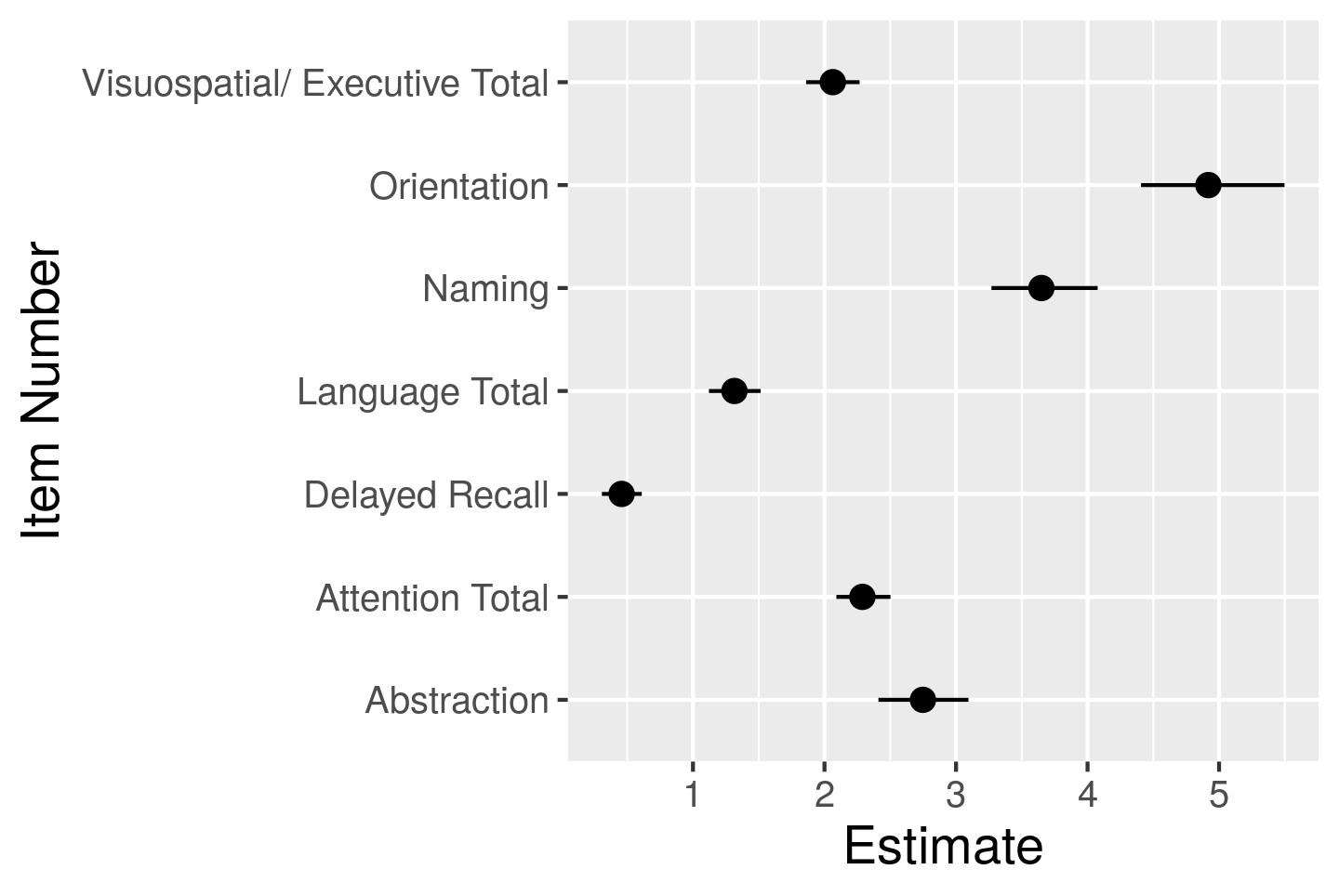 Figure 1: Item easiness for the 1PL model. MoCA Orientation are agreed by majority of patients (i.e., high score in that item) meanwhile Delayed Recall shows a disagreement amongst SLE patients).
Figure 1: Item easiness for the 1PL model. MoCA Orientation are agreed by majority of patients (i.e., high score in that item) meanwhile Delayed Recall shows a disagreement amongst SLE patients).
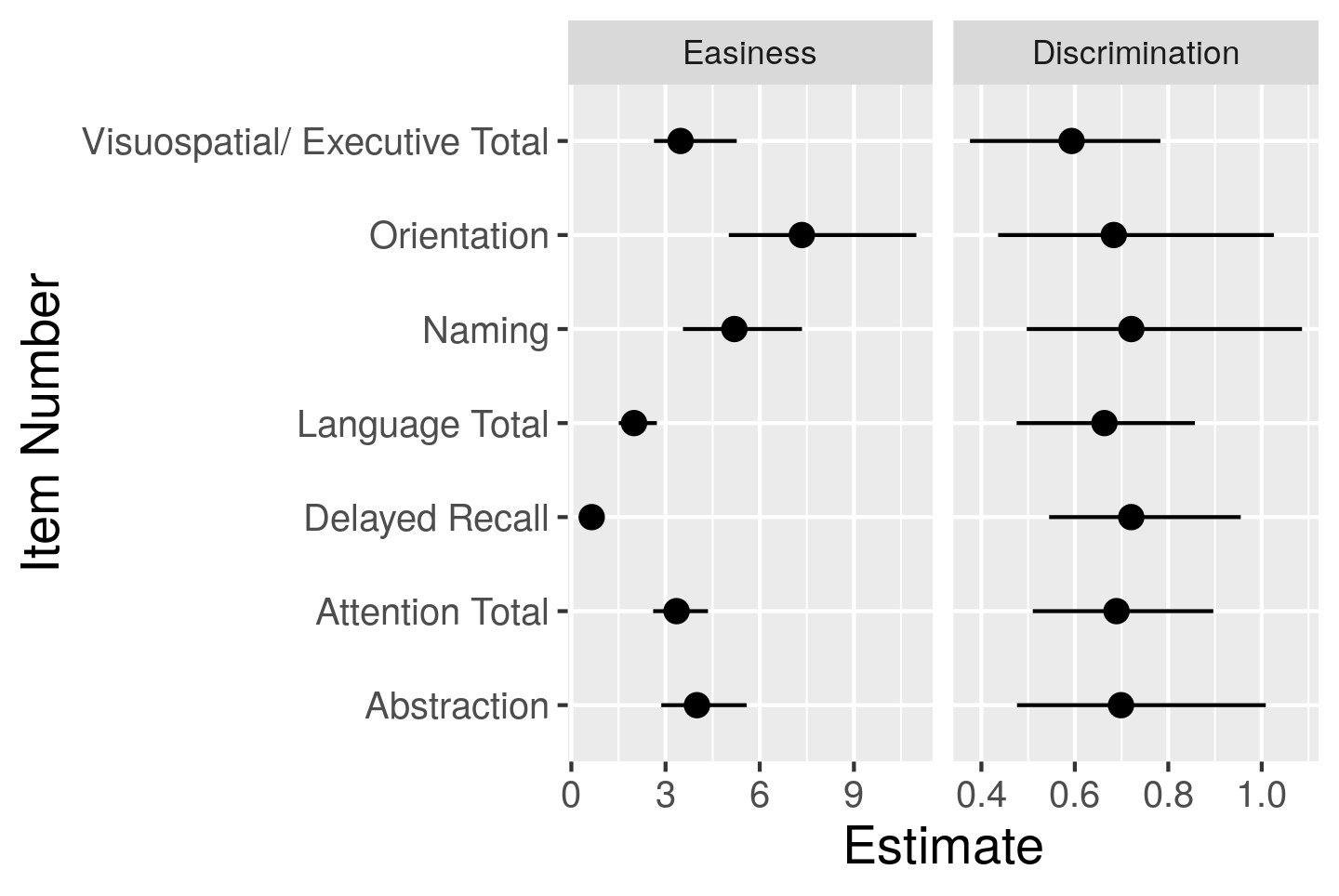 Figure 2: Item easiness and discrimination for the 2PL model. The figure on the right shows that the weights are similar across items (i.e. same discrimination) and therefore there is more uncertainty on the estimates of the easiness parameters (left figure).
Figure 2: Item easiness and discrimination for the 2PL model. The figure on the right shows that the weights are similar across items (i.e. same discrimination) and therefore there is more uncertainty on the estimates of the easiness parameters (left figure).
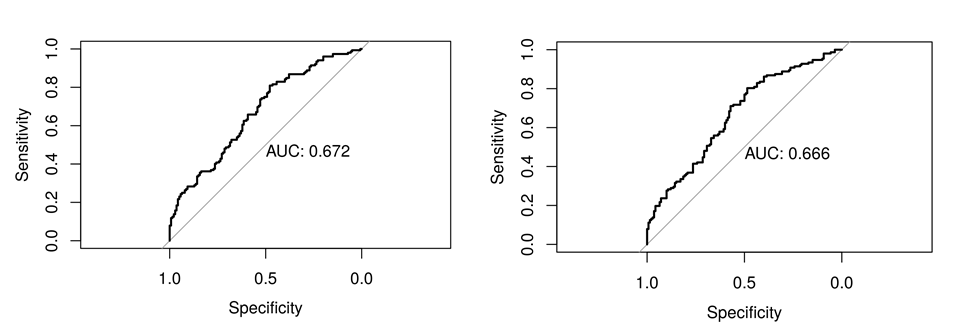 Figure 3: ROC curves for 1PL (left) and 2PL (right) models.
Figure 3: ROC curves for 1PL (left) and 2PL (right) models.
Disclosures: J. Diaz-Martinez, None; M. Barraclough, None; K. Yuen, None; O. Tayer-Shifman, None; A. Knight, None; K. Bingham, None; J. Su, None; M. Kakvan, None; C. Munoz, None; M. Tartaglia, None; L. Ruttan, None; J. Wither, AstraZeneca, Pfizer; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; D. Bonilla, None; S. Appenzeller, None; P. Katz, None; D. Beaton, None; R. Green, None; Z. Touma, None.
Background/Purpose: Cognitive impairment (CI) is one of the most common manifestations of neuropsychiatric syndromes in patients with SLE, with a prevalence ranging from 20% to 80% and a pooled prevalence of 38%. Previous studies have assessed the utility of the Montreal Cognitive Assessment (MoCA) as a screening tool for CI in SLE patients compared to the American College of Rheumatology neuropsychological battery (ACR-NB), where the performance of the MoCA in the identification of CI has been historically evaluated using a total score of 26 as the threshold. Instead of using this threshold, this study fits an item response theory (IRT) model to the MoCA questionnaire and compares its results to the ACR-NB to assess the utility of the MoCA as a stand-alone screening test.
Methods: Adult SLE patients who attended the University of Toronto Lupus Clinic between July 2016 and March 2020 (excluding those with learning disabilities and less than semi-fluent English language skills) were administered the MoCA followed by the ACR-NB. Patients were classified as having CI if they had a z-score of ≤-1.5 in ≥2 domains of the 6 domains of the ACR-NB, and otherwise non-CI. Using a Bayesian framework, different IRT models with a beta-binomial distribution were used in this analysis. IRT models aim to model persons' responses on a set of items measuring one or more latent constructs, which in our case this latent construct corresponds to CI in SLE patients. Specifically, we fitted 1PL and 2PL models, where the 1PL model estimates the "easiness" of each item (i.e., agreement across patients) and the 2PL adds a discrimination parameter for each item (i.e., not assuming that discriminations are to be constant across items). The performance of both IRT models using the MoCA in the identification of CI as classified by the ACR-NB was evaluated using ROC curve analysis and AUC calculation.
Results: A total of 276 SLE patients were enrolled; 88.8% were female and mean age and mean SLE disease duration at study visit were 41.3 ± 12.1 and 14.4 ± 10.1 years, respectively. Based on the ACR-NB, 129 patients (47%) were classified as having CI, 85 (31%) as undetermined CI, and 62 (22%) as non-CI patients. The 1PL model showed that some items (e.g., Orientation) were consistent amongst most individuals (agreement amongst patients) and thus had strongly positive easiness parameters (Figure 1), while other items (e.g., Delayed Recall) were mostly rejected and thus had a low easiness parameter. For the 2PL model, the easiness parameters displayed on the left-hand side of (Figure 2) still showed a similar pattern as in the 1PL, although their estimates were more spread out because of the discrimination estimates being less than 1. CI was poorly predicted by both IRT models (Figure 3) based on MoCA with an area under curve (AUC) of 67.2% for the 1PL model and 66.6% by the 2PL model.
Conclusion: This study found that the utility of the MoCA in screening for CI in SLE patients is limited. Future research should focus on other IRT models with different response distributions in order to determine if the accuracy of the MoCA could be improved by deriving a different weight score for each domain.
 Figure 1: Item easiness for the 1PL model. MoCA Orientation are agreed by majority of patients (i.e., high score in that item) meanwhile Delayed Recall shows a disagreement amongst SLE patients).
Figure 1: Item easiness for the 1PL model. MoCA Orientation are agreed by majority of patients (i.e., high score in that item) meanwhile Delayed Recall shows a disagreement amongst SLE patients). Figure 2: Item easiness and discrimination for the 2PL model. The figure on the right shows that the weights are similar across items (i.e. same discrimination) and therefore there is more uncertainty on the estimates of the easiness parameters (left figure).
Figure 2: Item easiness and discrimination for the 2PL model. The figure on the right shows that the weights are similar across items (i.e. same discrimination) and therefore there is more uncertainty on the estimates of the easiness parameters (left figure). Figure 3: ROC curves for 1PL (left) and 2PL (right) models.
Figure 3: ROC curves for 1PL (left) and 2PL (right) models.Disclosures: J. Diaz-Martinez, None; M. Barraclough, None; K. Yuen, None; O. Tayer-Shifman, None; A. Knight, None; K. Bingham, None; J. Su, None; M. Kakvan, None; C. Munoz, None; M. Tartaglia, None; L. Ruttan, None; J. Wither, AstraZeneca, Pfizer; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; D. Bonilla, None; S. Appenzeller, None; P. Katz, None; D. Beaton, None; R. Green, None; Z. Touma, None.

