Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1520: Rituximab and Tocilizumab and Their Effect on Lung Disease Progression in Scleroderma. a Retrospective Cohort Study at a Single Centre
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- VO
Voon Ong, PhD, FRCP
UCL Medical School Royal Free Campus
London, United Kingdom
Abstract Poster Presenter(s)
Nina Goldman1, Aoife Tynan2, Shivani Shah2, Claire Beesley1, Rizgar Mageed3, Chris Denton1 and Voon Ong4, 1University College London, London, United Kingdom, 2Division of Medicine, Department of Rheumatology, University College London, London, United Kingdom, 3Experimental Medicine and Rheumatology, William Harvey Research Institute, Queen Mary University, London, United Kingdom, 4UCL Medical School Royal Free Campus, London, United Kingdom
Background/Purpose: Interstitial lung disease (ILD) is one of the leading causes of mortality in systemic sclerosis (SSc). Emerging evidence suggests a beneficial effect of tocilizumab and rituximab on lung function however comparative data outside the arena of clinical trials remains limited. Deciding when and who to treat with specific treatments remains challenging.
Methods: We performed a retrospective analysis of all SSc patients attending a single specialist centre who had received rituximab, tocilizumab or both treatments. Demographic, clinical and laboratory data was collected along with lung function. Patients were excluded if lung function pre and/or post therapy was not available. Data was analysed based on anti-topoisomerase I antibody (ATA) status. Wilcoxon T test and Fishers exact test were used.
Results: 129 patients were included, of which 87 received rituximab, 32 tocilizumab and 10 both therapies. 8 of 10 (80%) patients who had received both therapies received rituximab prior to tocilizumab. 76 (58.9%) patients had diffuse SSc and 102 (79%) had ILD. Concurrent mycophenolate mofetil (MMF) was prescribed for 52 (53.6%) patients with rituximab and 17 (40.5%) patients with tocilizumab. Median pre-treatment lung function percentage forced vital capacity (%FVC) and percentage transfer factor (%DLCO) were 67.9% and 42.5% and 85.5% and 59.8% for rituximab and tocilizumab respectively. Median change %FVC and %DLCO pre- and post-treatment were +0.35% and -0.8% for rituximab and -0.9% and +0.2% for tocilizumab. The majority of patients improved or remained stable with either treatment and fewer patients on rituximab demonstrated FVC decline compared with tocilizumab (Figure 1). The effect from rituximab was not affected by ATA status in contrast, ATA positive patients were more likely to respond to tocilizumab (p = 0.0073) (Median FVC change: tocilizumab; + 60ml ATA positive vs -110ml ATA negative, rituximab; 0ml ATA positive vs 0ml ATA negative, Figure 2). Disease duration and CRP had no effect on treatment response for either therapy.
Conclusion: Our retrospective cohort provides real-life data supporting the use of both rituximab and tocilizumab to stabilise ILD in SSc. Differential response based on autoantibody specificity and clinical parameters may help optimise patient selection for biological therapy in SSc-ILD.
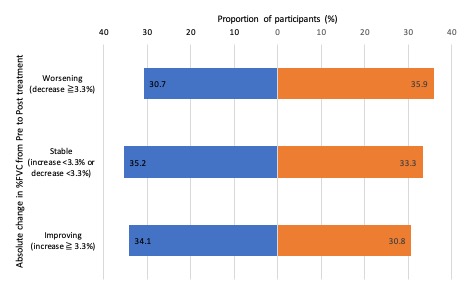 Figure 1. Change in absolute %FVC pre and post-treatment with rituximab or tocilizumab
Figure 1. Change in absolute %FVC pre and post-treatment with rituximab or tocilizumab
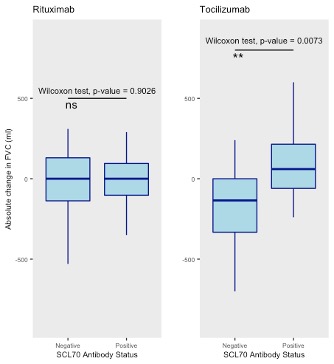 Figure 2: Absolute change in FVC (ml) for rituximab and tocilizumab treatment based on ATA antibody status
Figure 2: Absolute change in FVC (ml) for rituximab and tocilizumab treatment based on ATA antibody status
Disclosures: N. Goldman, None; A. Tynan, None; S. Shah, None; C. Beesley, None; R. Mageed, None; C. Denton, Boehringer-Ingelheim, Roche, GlaxoSmithKlein(GSK), Horizon; V. Ong, None.
Background/Purpose: Interstitial lung disease (ILD) is one of the leading causes of mortality in systemic sclerosis (SSc). Emerging evidence suggests a beneficial effect of tocilizumab and rituximab on lung function however comparative data outside the arena of clinical trials remains limited. Deciding when and who to treat with specific treatments remains challenging.
Methods: We performed a retrospective analysis of all SSc patients attending a single specialist centre who had received rituximab, tocilizumab or both treatments. Demographic, clinical and laboratory data was collected along with lung function. Patients were excluded if lung function pre and/or post therapy was not available. Data was analysed based on anti-topoisomerase I antibody (ATA) status. Wilcoxon T test and Fishers exact test were used.
Results: 129 patients were included, of which 87 received rituximab, 32 tocilizumab and 10 both therapies. 8 of 10 (80%) patients who had received both therapies received rituximab prior to tocilizumab. 76 (58.9%) patients had diffuse SSc and 102 (79%) had ILD. Concurrent mycophenolate mofetil (MMF) was prescribed for 52 (53.6%) patients with rituximab and 17 (40.5%) patients with tocilizumab. Median pre-treatment lung function percentage forced vital capacity (%FVC) and percentage transfer factor (%DLCO) were 67.9% and 42.5% and 85.5% and 59.8% for rituximab and tocilizumab respectively. Median change %FVC and %DLCO pre- and post-treatment were +0.35% and -0.8% for rituximab and -0.9% and +0.2% for tocilizumab. The majority of patients improved or remained stable with either treatment and fewer patients on rituximab demonstrated FVC decline compared with tocilizumab (Figure 1). The effect from rituximab was not affected by ATA status in contrast, ATA positive patients were more likely to respond to tocilizumab (p = 0.0073) (Median FVC change: tocilizumab; + 60ml ATA positive vs -110ml ATA negative, rituximab; 0ml ATA positive vs 0ml ATA negative, Figure 2). Disease duration and CRP had no effect on treatment response for either therapy.
Conclusion: Our retrospective cohort provides real-life data supporting the use of both rituximab and tocilizumab to stabilise ILD in SSc. Differential response based on autoantibody specificity and clinical parameters may help optimise patient selection for biological therapy in SSc-ILD.
 Figure 1. Change in absolute %FVC pre and post-treatment with rituximab or tocilizumab
Figure 1. Change in absolute %FVC pre and post-treatment with rituximab or tocilizumab Figure 2: Absolute change in FVC (ml) for rituximab and tocilizumab treatment based on ATA antibody status
Figure 2: Absolute change in FVC (ml) for rituximab and tocilizumab treatment based on ATA antibody status Disclosures: N. Goldman, None; A. Tynan, None; S. Shah, None; C. Beesley, None; R. Mageed, None; C. Denton, Boehringer-Ingelheim, Roche, GlaxoSmithKlein(GSK), Horizon; V. Ong, None.

