Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1150–1165) Spondyloarthritis Including PsA – Basic Science Poster
1150: Spatial Transcriptomics Stratifies Health and Psoriatic Disease Severity by Emergent Cellular Ecosystems
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Rochelle Castillo, MD, MS
NYU Langone Health
New York, NY, United States
Abstract Poster Presenter(s)
Rochelle Castillo1, Ikjot Sidhu1, Igor Dolgalev1, Ipsita Subudhi1, Di Yan1, Piotr Konieczny1, Brandon Hsieh1, Tinyi Chu2, Rebecca Haberman1, Shanmugapriya Selvaraj1, Tomoe Shiomi1, Rhina Medina1, Parvathy Vasudevanpillai Girija1, Adriana Heguy1, Cynthia Loomis1, Luis Chiriboga1, Shane Meehan1, Christopher Ritchlin3, Maria de la Luz Garcia-Hernandez4, John Carucci1, Andrea Neimann1, Shruti Naik1 and Jose Scher5, 1NYU Langone Health, New York, NY, 2Memorial Sloan Kettering Cancer Center, New York, NY, 3Allergy, Immunology and Rheumatology Division, University of Rochester Medical School, Canandaigua, NY, 4University of Rochester, West Henrietta, NY, 5New York University School of Medicine, New York, NY
Background/Purpose: The skin is recognized as a window into the immunopathogenic mechanisms driving the vast phenotypic spectrum of psoriatic disease.
Methods: To better decipher the cellular landscape of both healthy and psoriatic skin, we employed spatial transcriptomics (ST), a ground-breaking technology that precisely maps gene expression from histologically-intact tissue sections (Fig. 1A).
Results: Findings gleaned from computationally integrating our 23 matched lesional and non-lesional psoriatic and 7 healthy control samples with publicly-available single-cell ribonucleic acid (RNA) sequencing datasets established the ability of ST to recapitulate the tissue architecture of both healthy and inflamed skin (Fig. 1B) and highlighted topographic shifts in the immune cell milieu, from a predominantly perifollicular distribution in steady-state skin to the papillary and upper reticular dermis in psoriatic lesional skin. We also incidentally discovered that ST's ability to ascertain gene expression patterns from intact tissue rendered it particularly conducive to studying the transcriptome of lipid-laden cells such as dermal adipose tissue and sebaceous glands (Fig. 1C), whose expression profiles are typically lost in the process of tissue handling and dissociation for bulk and single-cell RNA seq. Unbiased clustering of pooled healthy and psoriatic samples identified two epidermal clusters and one dermal cluster that were differentially expanded in psoriatic lesional skin (p values ≤0.05) (Fig. 1D); pathway analysis of these clusters revealed enrichment of known psoriatic inflammatory pathways (Fig. 1E). Unsupervised classification of skin-limited psoriasis and psoriatic arthritis samples revealed stratification by cutaneous disease severity or Psoriasis Area and Severity Index (PASI) score and not by presence or absence of concomitant systemic/synovial disease (Fig. 1F). Remarkably, this PASI-dependent segregation was also evident in distal, non-lesional samples and was driven by the dermal macrophage and fibroblast cluster and the lymphatic endothelium (Fig. 2A). Inquiry into the mechanistic drivers of this observed stratification yielded enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (Fig. 2B). Finally, tissue scale computational cartography of gene expression revealed differences in regional enrichment of specific cell types across phenotypic groups, most notably upward extension of fibroblasts to the upper dermis in both lesional and non-lesional samples from mild psoriasis and restriction to the lower dermis in the moderate-to-severe psoriasis samples (Fig. 2C), suggesting that disease severity stratification may be driven by emergent cellular ecosystems in the upper dermis.
Conclusion: Thus, we have been able to successfully leverage ST integrated with independently-generated single-cell RNA seq data to spatially define the emergent cellular ecosystems of healthy and matched psoriatic lesional and non-lesional skin and in so doing, demonstrated the value of ST in unearthing the genetic groundwork at both the site of inflammation and in distal, clinically-uninvolved skin.
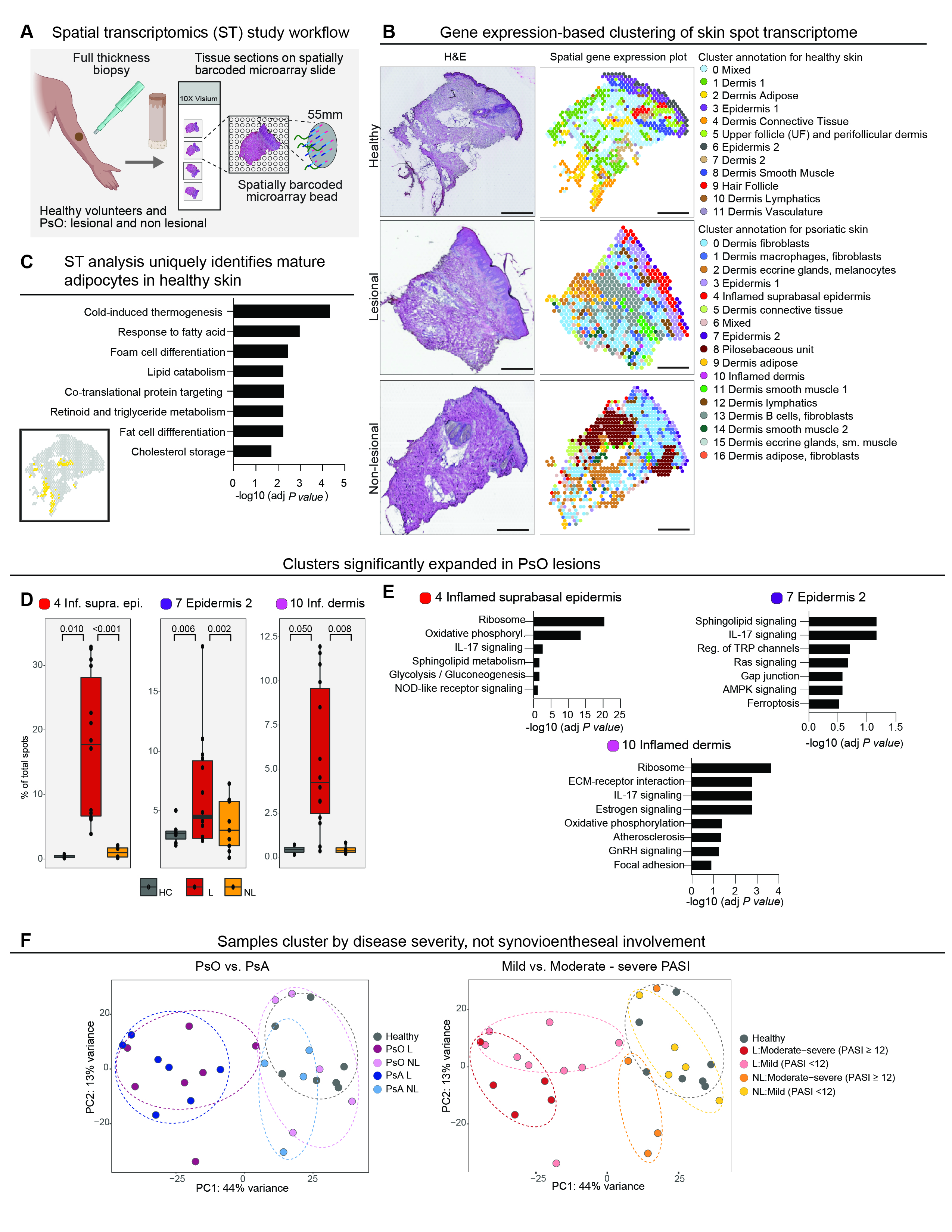 Fig. 1. (A) Schematic of spatial transcriptomics study workflow. Four mm skin punch biopsies were obtained from healthy volunteers (n=3) and lesional and non-lesional skin from patients with psoriatic disease (n=11). Ten micron-thick sections were then placed on capture areas on the ST microarray slide, each containing molecularly barcoded, spatially encoded spots with a diameter of 50 microns and a center-to-center distance of 100 microns. (B) Side-by-side comparison of a hematoxylin-eosin (H&E) stained section of representative healthy, lesional, and non-lesional skin samples and the corresponding ST plots showed concordance of unbiased gene expression-based clustering with histologic tissue architecture. (C) Pathway analysis of the adipose cluster in healthy skin (cluster 2) confirmed upregulation of lipid-associated processes. Inset: Spots corresponding to the adipose cluster highlighted in yellow. (D) Wilcoxon rank sum test (results displayed as box plots) yielded statistically significant expansion of three clusters in lesional skin compared to both non-lesional and healthy skin – inflamed suprabasal epidermis (cluster 4), epidermis 2 (cluster 7), and inflamed dermis (cluster 10). HC=healthy control, L=lesional psoriatic skin, NL=non-lesional psoriatic skin. (E) Pathways enriched in clusters 4, 7, and 10. (F) Principal component analysis (PCA) plots demonstrating segregation of samples by severity of cutaneous disease in both lesional and non-lesional samples along the first principal component (right) that was not seen in the samples categorized according to presence or absence of arthritis (left). PsA=psoriatic arthritis, PsO=skin-limited psoriasis.
Fig. 1. (A) Schematic of spatial transcriptomics study workflow. Four mm skin punch biopsies were obtained from healthy volunteers (n=3) and lesional and non-lesional skin from patients with psoriatic disease (n=11). Ten micron-thick sections were then placed on capture areas on the ST microarray slide, each containing molecularly barcoded, spatially encoded spots with a diameter of 50 microns and a center-to-center distance of 100 microns. (B) Side-by-side comparison of a hematoxylin-eosin (H&E) stained section of representative healthy, lesional, and non-lesional skin samples and the corresponding ST plots showed concordance of unbiased gene expression-based clustering with histologic tissue architecture. (C) Pathway analysis of the adipose cluster in healthy skin (cluster 2) confirmed upregulation of lipid-associated processes. Inset: Spots corresponding to the adipose cluster highlighted in yellow. (D) Wilcoxon rank sum test (results displayed as box plots) yielded statistically significant expansion of three clusters in lesional skin compared to both non-lesional and healthy skin – inflamed suprabasal epidermis (cluster 4), epidermis 2 (cluster 7), and inflamed dermis (cluster 10). HC=healthy control, L=lesional psoriatic skin, NL=non-lesional psoriatic skin. (E) Pathways enriched in clusters 4, 7, and 10. (F) Principal component analysis (PCA) plots demonstrating segregation of samples by severity of cutaneous disease in both lesional and non-lesional samples along the first principal component (right) that was not seen in the samples categorized according to presence or absence of arthritis (left). PsA=psoriatic arthritis, PsO=skin-limited psoriasis.
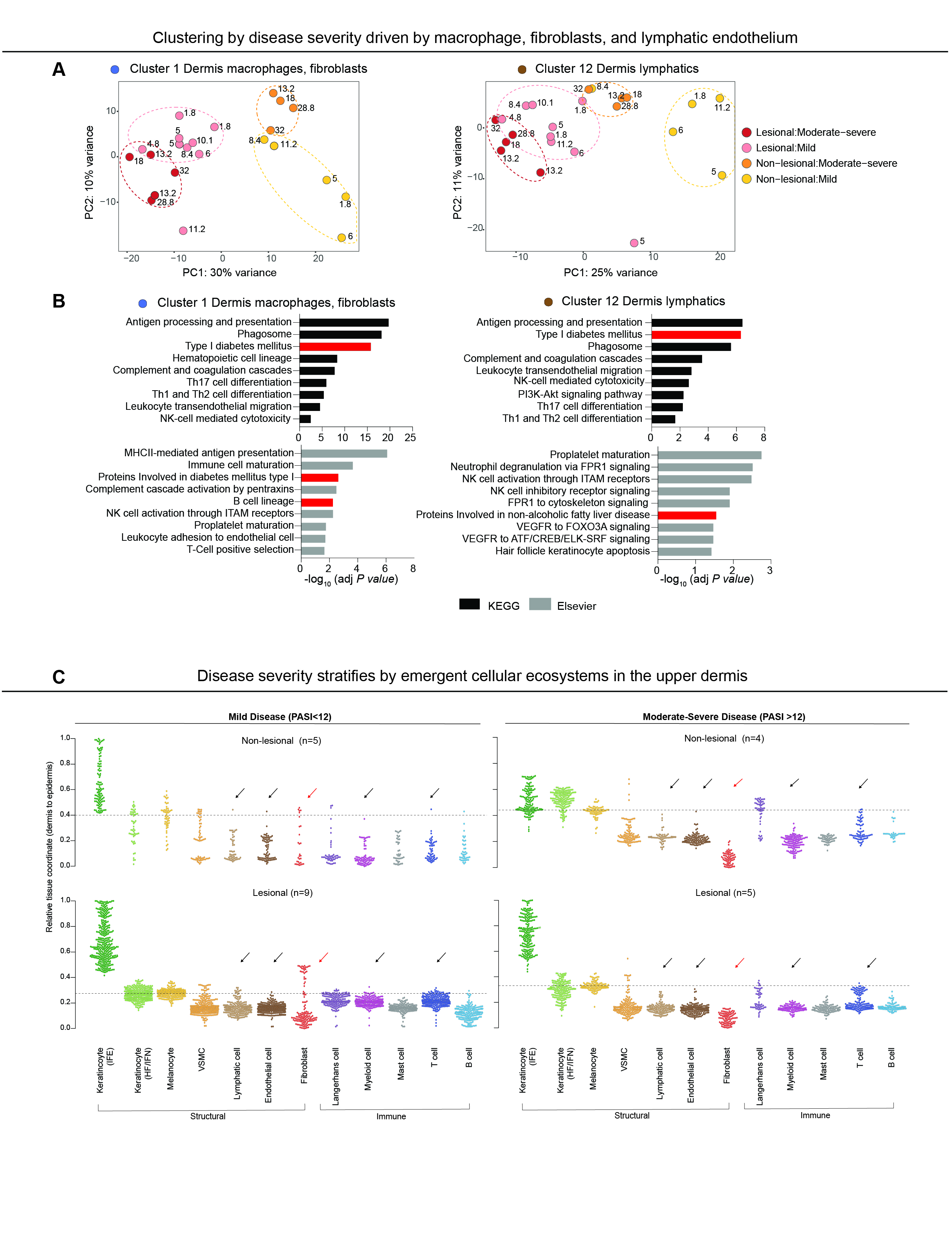 Fig. 2. (A) PCA of lesional and non-lesional samples colored by disease severity in spatial clusters 1 (left) and 12 (right) revealed more discrete clustering. (B) Pathways significantly enriched in clusters 1 (left) and 12 (right) showed enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (highlighted in red). (C) SpaceFold one dimension projection of cell distribution from an independently-generated single-cell RNA seq data set on aggregated ST lesional and non-lesional samples from mild (PASI-low) and moderate-severe (PASI-high) samples. Y-axis represents tissue position, starting with the lower dermis marked as position 0 to suprabasal epidermis marked as position 1. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Fibroblast signatures (red arrows) were largely relegated to the lower dermis in the PASI-high group, but extended to the upper dermis in the PASI-low group. This striking difference in fibroblast localization was also noted in non-lesional PASI-high vs. PASI-low groups. In addition to fibroblasts, lymphatic, endothelial, myeloid, and T cells signatures (black arrows) were also observed in the upper dermis of lesional PASI-low samples, but were much lower in the dermis of PASI-low non-lesional and all samples in the PASI-high group. Interfollicular epidermis (IFE), hair follicle and infundibulum (HF/IFN), n= number of individual biopsies.
Fig. 2. (A) PCA of lesional and non-lesional samples colored by disease severity in spatial clusters 1 (left) and 12 (right) revealed more discrete clustering. (B) Pathways significantly enriched in clusters 1 (left) and 12 (right) showed enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (highlighted in red). (C) SpaceFold one dimension projection of cell distribution from an independently-generated single-cell RNA seq data set on aggregated ST lesional and non-lesional samples from mild (PASI-low) and moderate-severe (PASI-high) samples. Y-axis represents tissue position, starting with the lower dermis marked as position 0 to suprabasal epidermis marked as position 1. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Fibroblast signatures (red arrows) were largely relegated to the lower dermis in the PASI-high group, but extended to the upper dermis in the PASI-low group. This striking difference in fibroblast localization was also noted in non-lesional PASI-high vs. PASI-low groups. In addition to fibroblasts, lymphatic, endothelial, myeloid, and T cells signatures (black arrows) were also observed in the upper dermis of lesional PASI-low samples, but were much lower in the dermis of PASI-low non-lesional and all samples in the PASI-high group. Interfollicular epidermis (IFE), hair follicle and infundibulum (HF/IFN), n= number of individual biopsies.
Disclosures: R. Castillo, None; I. Sidhu, None; I. Dolgalev, None; I. Subudhi, None; D. Yan, None; P. Konieczny, None; B. Hsieh, None; T. Chu, None; R. Haberman, None; S. Selvaraj, None; T. Shiomi, None; R. Medina, None; P. Vasudevanpillai Girija, None; A. Heguy, None; C. Loomis, None; L. Chiriboga, None; S. Meehan, None; C. Ritchlin, UCB, AbbVie, Eli Lilly, Pfizer Inc, Novartis, Janssen, Bristol-Myers Squibb; M. Garcia-Hernandez, None; J. Carucci, Genentech; A. Neimann, Janssen, UCB, AbbVie/Abbott, Bristol-Myers Squibb(BMS); S. Naik, None; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB.
Background/Purpose: The skin is recognized as a window into the immunopathogenic mechanisms driving the vast phenotypic spectrum of psoriatic disease.
Methods: To better decipher the cellular landscape of both healthy and psoriatic skin, we employed spatial transcriptomics (ST), a ground-breaking technology that precisely maps gene expression from histologically-intact tissue sections (Fig. 1A).
Results: Findings gleaned from computationally integrating our 23 matched lesional and non-lesional psoriatic and 7 healthy control samples with publicly-available single-cell ribonucleic acid (RNA) sequencing datasets established the ability of ST to recapitulate the tissue architecture of both healthy and inflamed skin (Fig. 1B) and highlighted topographic shifts in the immune cell milieu, from a predominantly perifollicular distribution in steady-state skin to the papillary and upper reticular dermis in psoriatic lesional skin. We also incidentally discovered that ST's ability to ascertain gene expression patterns from intact tissue rendered it particularly conducive to studying the transcriptome of lipid-laden cells such as dermal adipose tissue and sebaceous glands (Fig. 1C), whose expression profiles are typically lost in the process of tissue handling and dissociation for bulk and single-cell RNA seq. Unbiased clustering of pooled healthy and psoriatic samples identified two epidermal clusters and one dermal cluster that were differentially expanded in psoriatic lesional skin (p values ≤0.05) (Fig. 1D); pathway analysis of these clusters revealed enrichment of known psoriatic inflammatory pathways (Fig. 1E). Unsupervised classification of skin-limited psoriasis and psoriatic arthritis samples revealed stratification by cutaneous disease severity or Psoriasis Area and Severity Index (PASI) score and not by presence or absence of concomitant systemic/synovial disease (Fig. 1F). Remarkably, this PASI-dependent segregation was also evident in distal, non-lesional samples and was driven by the dermal macrophage and fibroblast cluster and the lymphatic endothelium (Fig. 2A). Inquiry into the mechanistic drivers of this observed stratification yielded enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (Fig. 2B). Finally, tissue scale computational cartography of gene expression revealed differences in regional enrichment of specific cell types across phenotypic groups, most notably upward extension of fibroblasts to the upper dermis in both lesional and non-lesional samples from mild psoriasis and restriction to the lower dermis in the moderate-to-severe psoriasis samples (Fig. 2C), suggesting that disease severity stratification may be driven by emergent cellular ecosystems in the upper dermis.
Conclusion: Thus, we have been able to successfully leverage ST integrated with independently-generated single-cell RNA seq data to spatially define the emergent cellular ecosystems of healthy and matched psoriatic lesional and non-lesional skin and in so doing, demonstrated the value of ST in unearthing the genetic groundwork at both the site of inflammation and in distal, clinically-uninvolved skin.
 Fig. 1. (A) Schematic of spatial transcriptomics study workflow. Four mm skin punch biopsies were obtained from healthy volunteers (n=3) and lesional and non-lesional skin from patients with psoriatic disease (n=11). Ten micron-thick sections were then placed on capture areas on the ST microarray slide, each containing molecularly barcoded, spatially encoded spots with a diameter of 50 microns and a center-to-center distance of 100 microns. (B) Side-by-side comparison of a hematoxylin-eosin (H&E) stained section of representative healthy, lesional, and non-lesional skin samples and the corresponding ST plots showed concordance of unbiased gene expression-based clustering with histologic tissue architecture. (C) Pathway analysis of the adipose cluster in healthy skin (cluster 2) confirmed upregulation of lipid-associated processes. Inset: Spots corresponding to the adipose cluster highlighted in yellow. (D) Wilcoxon rank sum test (results displayed as box plots) yielded statistically significant expansion of three clusters in lesional skin compared to both non-lesional and healthy skin – inflamed suprabasal epidermis (cluster 4), epidermis 2 (cluster 7), and inflamed dermis (cluster 10). HC=healthy control, L=lesional psoriatic skin, NL=non-lesional psoriatic skin. (E) Pathways enriched in clusters 4, 7, and 10. (F) Principal component analysis (PCA) plots demonstrating segregation of samples by severity of cutaneous disease in both lesional and non-lesional samples along the first principal component (right) that was not seen in the samples categorized according to presence or absence of arthritis (left). PsA=psoriatic arthritis, PsO=skin-limited psoriasis.
Fig. 1. (A) Schematic of spatial transcriptomics study workflow. Four mm skin punch biopsies were obtained from healthy volunteers (n=3) and lesional and non-lesional skin from patients with psoriatic disease (n=11). Ten micron-thick sections were then placed on capture areas on the ST microarray slide, each containing molecularly barcoded, spatially encoded spots with a diameter of 50 microns and a center-to-center distance of 100 microns. (B) Side-by-side comparison of a hematoxylin-eosin (H&E) stained section of representative healthy, lesional, and non-lesional skin samples and the corresponding ST plots showed concordance of unbiased gene expression-based clustering with histologic tissue architecture. (C) Pathway analysis of the adipose cluster in healthy skin (cluster 2) confirmed upregulation of lipid-associated processes. Inset: Spots corresponding to the adipose cluster highlighted in yellow. (D) Wilcoxon rank sum test (results displayed as box plots) yielded statistically significant expansion of three clusters in lesional skin compared to both non-lesional and healthy skin – inflamed suprabasal epidermis (cluster 4), epidermis 2 (cluster 7), and inflamed dermis (cluster 10). HC=healthy control, L=lesional psoriatic skin, NL=non-lesional psoriatic skin. (E) Pathways enriched in clusters 4, 7, and 10. (F) Principal component analysis (PCA) plots demonstrating segregation of samples by severity of cutaneous disease in both lesional and non-lesional samples along the first principal component (right) that was not seen in the samples categorized according to presence or absence of arthritis (left). PsA=psoriatic arthritis, PsO=skin-limited psoriasis. Fig. 2. (A) PCA of lesional and non-lesional samples colored by disease severity in spatial clusters 1 (left) and 12 (right) revealed more discrete clustering. (B) Pathways significantly enriched in clusters 1 (left) and 12 (right) showed enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (highlighted in red). (C) SpaceFold one dimension projection of cell distribution from an independently-generated single-cell RNA seq data set on aggregated ST lesional and non-lesional samples from mild (PASI-low) and moderate-severe (PASI-high) samples. Y-axis represents tissue position, starting with the lower dermis marked as position 0 to suprabasal epidermis marked as position 1. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Fibroblast signatures (red arrows) were largely relegated to the lower dermis in the PASI-high group, but extended to the upper dermis in the PASI-low group. This striking difference in fibroblast localization was also noted in non-lesional PASI-high vs. PASI-low groups. In addition to fibroblasts, lymphatic, endothelial, myeloid, and T cells signatures (black arrows) were also observed in the upper dermis of lesional PASI-low samples, but were much lower in the dermis of PASI-low non-lesional and all samples in the PASI-high group. Interfollicular epidermis (IFE), hair follicle and infundibulum (HF/IFN), n= number of individual biopsies.
Fig. 2. (A) PCA of lesional and non-lesional samples colored by disease severity in spatial clusters 1 (left) and 12 (right) revealed more discrete clustering. (B) Pathways significantly enriched in clusters 1 (left) and 12 (right) showed enrichment of pathways associated with key T cell and innate immune cell activation, B cells, and metabolic dysfunction (highlighted in red). (C) SpaceFold one dimension projection of cell distribution from an independently-generated single-cell RNA seq data set on aggregated ST lesional and non-lesional samples from mild (PASI-low) and moderate-severe (PASI-high) samples. Y-axis represents tissue position, starting with the lower dermis marked as position 0 to suprabasal epidermis marked as position 1. Dashed line represents epidermal-dermal junction, discerned by cell types in the basal epidermal layer (melanocytes and Langerhans cells). Fibroblast signatures (red arrows) were largely relegated to the lower dermis in the PASI-high group, but extended to the upper dermis in the PASI-low group. This striking difference in fibroblast localization was also noted in non-lesional PASI-high vs. PASI-low groups. In addition to fibroblasts, lymphatic, endothelial, myeloid, and T cells signatures (black arrows) were also observed in the upper dermis of lesional PASI-low samples, but were much lower in the dermis of PASI-low non-lesional and all samples in the PASI-high group. Interfollicular epidermis (IFE), hair follicle and infundibulum (HF/IFN), n= number of individual biopsies.Disclosures: R. Castillo, None; I. Sidhu, None; I. Dolgalev, None; I. Subudhi, None; D. Yan, None; P. Konieczny, None; B. Hsieh, None; T. Chu, None; R. Haberman, None; S. Selvaraj, None; T. Shiomi, None; R. Medina, None; P. Vasudevanpillai Girija, None; A. Heguy, None; C. Loomis, None; L. Chiriboga, None; S. Meehan, None; C. Ritchlin, UCB, AbbVie, Eli Lilly, Pfizer Inc, Novartis, Janssen, Bristol-Myers Squibb; M. Garcia-Hernandez, None; J. Carucci, Genentech; A. Neimann, Janssen, UCB, AbbVie/Abbott, Bristol-Myers Squibb(BMS); S. Naik, None; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB.

