Back
Poster Session C
Genetics, genomics and proteomics
Session: (1118–1149) Genetics, Genomics and Proteomics Poster
1135: Urine Proteomics Implicate Complement C3 and Factor I in Lupus Nephritis Patients with Interstitial Fibrosis and Tubular Atrophy
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SW
Shudan Wang, MD, MS
Albert Einstein College of Medicine
New York, NY, United States
Abstract Poster Presenter(s)
Shudan Wang1, Anna Broder2, Daming Shao3, Vartika Kesarwani4, Brianna Lally5, Masako Suzuki6, J. Michelle Kahlenberg7, Jennifer Aguilan6 and Simone Sidoli6, 1Montefiore Medical Center / Albert Einstein College of Medicine, New York, NY, 2Hackensack University Medical Center, Hackensack, NJ, 3Albert Einstein College of Medicine/Jacobi Medical Center, Bronx, NY, 4University of Connecticut, Farmington, CT, 5University of Wisconsin Hospitals and Clinics, Madison, WI, 6Albert Einstein College of Medicine, Bronx, NY, 7University of Michigan, Ann Arbor, MI
Background/Purpose: The complement system has an important and underrecognized role in mediating tubulointerstitial disease in lupus nephritis (LN). The omics big data allows for identifying novel biomarkers and potential therapeutic targets for tubulointerstitial disease in LN. This study used a proteomic approach to compare the urinary complement protein profiles between LN patients with moderate-to-severe interstitial fibrosis/tubular atrophy (IFTA) and none-to-mild IFTA to identify potential markers of tubulointerstitial fibrosis.
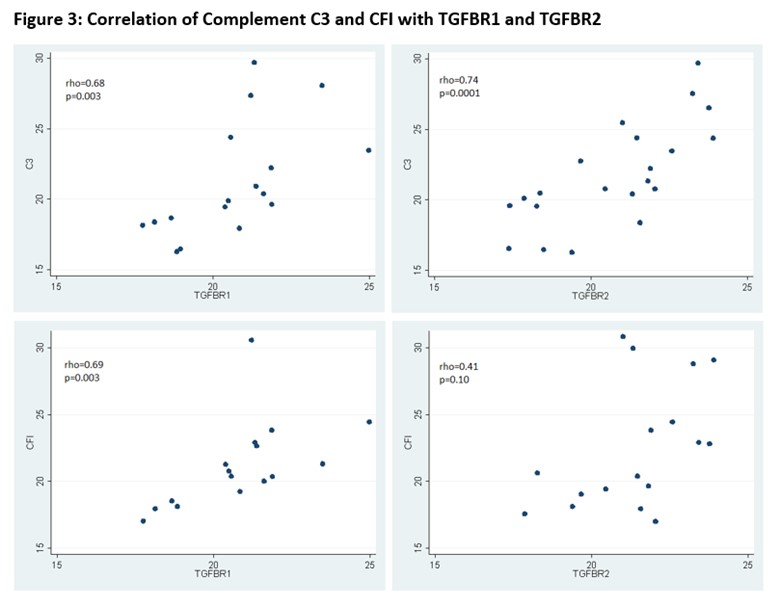
Methods: We quantified proteins in the urine samples from 46 adults and pediatric lupus patients with clinically indicated kidney biopsy performed between 2010 and 2019. Proteomics analysis was performed using mass spectrometry (Orbitrap Fusion Lumos, Thermo Scientific) and processed by the Proteome Discoverer. The detected urinary proteins were normalized by urine creatinine excretion. The proteins involved in the activation and inhibition of the complement pathways were identified and analyzed. The relative and absolute abundance of complement proteins was reported as median (interquartile range, IQR) and compared between patients with none-to-mild IFTA and moderate-to-severe IFTA on a logarithmic scale. IFTA was defined as none-to-mild if < 25% of the interstitium was affected by fibrosis and atrophy, and moderate-to-severe if ≥25% of the interstitium was affected. Urinary complement proteins enriched in patients with moderate-to-severe IFTA were correlated with transforming growth factor beta receptor 1 (TGFBR1) and transforming growth factor beta receptor 2 (TGFBR2), which are known mediators of kidney fibrosis.
Results: Forty-six patients with LN were included: 10 (21.7%) had moderate-to-severe IFTA and 36 had none-to-mild IFTA. Of the 1169 unique proteins identified, 18 were complement proteins and 4 were complement inhibitors. Patients with moderate-to-severe IFTA had a different clustering pattern from those with none-to-mild IFTA, suggesting there was a different expression profile of urinary complement proteins between the groups (Figure 1). Complement C3 and complement factor I (CFI) were significantly enriched in the moderate-to-severe IFTA versus none-to-mild IFTA group: C3 median (IQR) 24.4(23.5-25.5) vs. 20.2 (18.5–22.2), p= 0.02; CFI medium (IQR) 28.8 (21.8-30.6) vs. 20.4 (18.5-22.9), p=0.01) (Figure 2). No differences were found among the other complement proteins. Both C3 and CFI showed positive correlation with TGFBR1 while only C3 showed positive correlation with TGFBR2 (Figure 3).
Conclusion: This study is one of first to analyze the urinary complement profile in LN patients with moderate-to-severe IFTA and none-to-mild IFTA. This study identified urinary C3 and CFI as potential markers of tubulointerstitial fibrosis in LN. Further studies are needed to determine the pathogenic role of C3 and CFI in LN to identify new avenues for LN treatment.
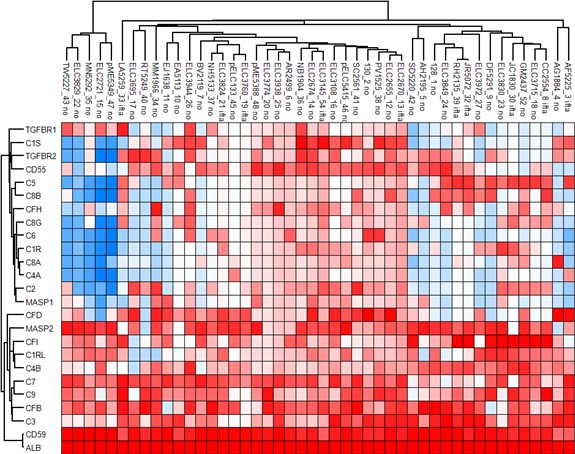 Figure 1: Heat map of urinary complement proteins
Figure 1: Heat map of urinary complement proteins
Red and blue colors indicate higher and lower expression respectively. Patients with moderate-to-severe IFTA are labeled as “ifta”, and patients with none-to-mild IFTA are labeled as “no”.
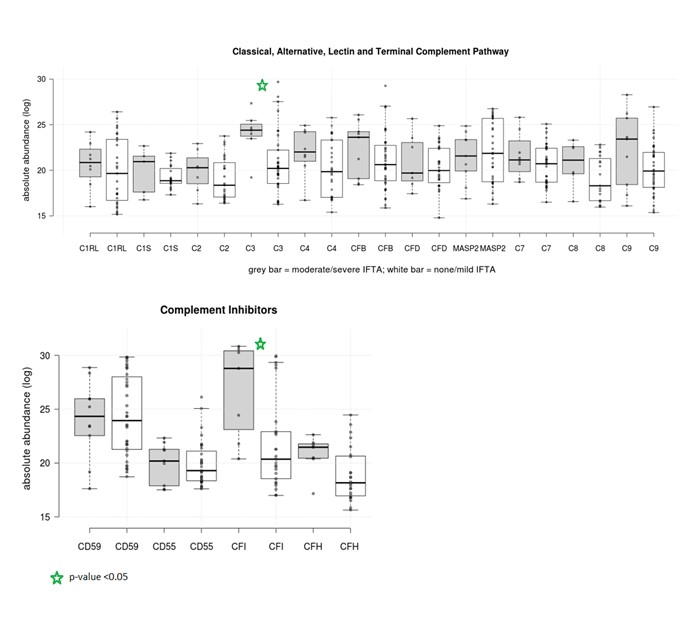 Figure 2: Comparison of Complement Components and Inhibitors between LN Patients with None-to-Mild IFTA and Moderate-to-Severe IFTA.
Figure 2: Comparison of Complement Components and Inhibitors between LN Patients with None-to-Mild IFTA and Moderate-to-Severe IFTA.
Dark grey box plots represent patients with moderate/severe IFTA. White box plots represent patients with none/mild IFTA.
Black line indicates median value in each group.
Green star indicates p-value < 0.05 by Wilcoxon Rank Sum test.
 References:
References:
1. Wang S, Wu M, Chiriboga L, et al. Membrane attack complex (MAC) deposition in renal tubules is associated with interstitial fibrosis and tubular atrophy: a pilot study. Lupus Sci Med. 2022 Jan;9(1).
2. Bao L, Wang Y, Haas M, et al. Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int. 2011 Sep;80(5):524-34.
Disclosures: S. Wang, None; A. Broder, None; D. Shao, None; V. Kesarwani, None; B. Lally, None; M. Suzuki, None; J. Kahlenberg, Q32 Bio, Celgene/Bristol Myers Squibb, Ventus Therapeutics, Rome Therapeutics, Janssen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Boehringer Ingelheim; J. Aguilan, None; S. Sidoli, None.
Background/Purpose: The complement system has an important and underrecognized role in mediating tubulointerstitial disease in lupus nephritis (LN). The omics big data allows for identifying novel biomarkers and potential therapeutic targets for tubulointerstitial disease in LN. This study used a proteomic approach to compare the urinary complement protein profiles between LN patients with moderate-to-severe interstitial fibrosis/tubular atrophy (IFTA) and none-to-mild IFTA to identify potential markers of tubulointerstitial fibrosis.
Methods: We quantified proteins in the urine samples from 46 adults and pediatric lupus patients with clinically indicated kidney biopsy performed between 2010 and 2019. Proteomics analysis was performed using mass spectrometry (Orbitrap Fusion Lumos, Thermo Scientific) and processed by the Proteome Discoverer. The detected urinary proteins were normalized by urine creatinine excretion. The proteins involved in the activation and inhibition of the complement pathways were identified and analyzed. The relative and absolute abundance of complement proteins was reported as median (interquartile range, IQR) and compared between patients with none-to-mild IFTA and moderate-to-severe IFTA on a logarithmic scale. IFTA was defined as none-to-mild if < 25% of the interstitium was affected by fibrosis and atrophy, and moderate-to-severe if ≥25% of the interstitium was affected. Urinary complement proteins enriched in patients with moderate-to-severe IFTA were correlated with transforming growth factor beta receptor 1 (TGFBR1) and transforming growth factor beta receptor 2 (TGFBR2), which are known mediators of kidney fibrosis.
Results: Forty-six patients with LN were included: 10 (21.7%) had moderate-to-severe IFTA and 36 had none-to-mild IFTA. Of the 1169 unique proteins identified, 18 were complement proteins and 4 were complement inhibitors. Patients with moderate-to-severe IFTA had a different clustering pattern from those with none-to-mild IFTA, suggesting there was a different expression profile of urinary complement proteins between the groups (Figure 1). Complement C3 and complement factor I (CFI) were significantly enriched in the moderate-to-severe IFTA versus none-to-mild IFTA group: C3 median (IQR) 24.4(23.5-25.5) vs. 20.2 (18.5–22.2), p= 0.02; CFI medium (IQR) 28.8 (21.8-30.6) vs. 20.4 (18.5-22.9), p=0.01) (Figure 2). No differences were found among the other complement proteins. Both C3 and CFI showed positive correlation with TGFBR1 while only C3 showed positive correlation with TGFBR2 (Figure 3).
Conclusion: This study is one of first to analyze the urinary complement profile in LN patients with moderate-to-severe IFTA and none-to-mild IFTA. This study identified urinary C3 and CFI as potential markers of tubulointerstitial fibrosis in LN. Further studies are needed to determine the pathogenic role of C3 and CFI in LN to identify new avenues for LN treatment.
 Figure 1: Heat map of urinary complement proteins
Figure 1: Heat map of urinary complement proteins Red and blue colors indicate higher and lower expression respectively. Patients with moderate-to-severe IFTA are labeled as “ifta”, and patients with none-to-mild IFTA are labeled as “no”.
 Figure 2: Comparison of Complement Components and Inhibitors between LN Patients with None-to-Mild IFTA and Moderate-to-Severe IFTA.
Figure 2: Comparison of Complement Components and Inhibitors between LN Patients with None-to-Mild IFTA and Moderate-to-Severe IFTA. Dark grey box plots represent patients with moderate/severe IFTA. White box plots represent patients with none/mild IFTA.
Black line indicates median value in each group.
Green star indicates p-value < 0.05 by Wilcoxon Rank Sum test.
 References:
References: 1. Wang S, Wu M, Chiriboga L, et al. Membrane attack complex (MAC) deposition in renal tubules is associated with interstitial fibrosis and tubular atrophy: a pilot study. Lupus Sci Med. 2022 Jan;9(1).
2. Bao L, Wang Y, Haas M, et al. Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int. 2011 Sep;80(5):524-34.
Disclosures: S. Wang, None; A. Broder, None; D. Shao, None; V. Kesarwani, None; B. Lally, None; M. Suzuki, None; J. Kahlenberg, Q32 Bio, Celgene/Bristol Myers Squibb, Ventus Therapeutics, Rome Therapeutics, Janssen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Boehringer Ingelheim; J. Aguilan, None; S. Sidoli, None.

