Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1574: Vasculitides as Medication-Associated Adverse Events Based on a National Database Reporting System
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.jpg)
Alicia Rodriguez-Pla, MD, PhD, RhMSUS, MPH, FACP
UCSF Fresno
Fresno, CA, United States
Abstract Poster Presenter(s)
Alicia Rodriguez-Pla, Mayo Clinic Arizona, Scottsdale, AZ
Background/Purpose: Vasculitides have been reported as adverse events (AEs) related to a wide variety of medications. We aimed to analyze the vasculitides reported to a national AE spontaneous reporting system from October 2012 through September 2021.
Methods: All spontaneous reports of vasculitis related to any medications from October 1st, 2012, to September 30th, 2021, were retrieved from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database. We performed a descriptive analysis of demographics, medications, and type of vasculitis based on 25 Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) codes. Medications identified as the primary suspect in individual case safety reports (ICSRs) were retrieved by their product active ingredient. RStudio v1.4.1717 was used for general data analysis and the R package openEBGM v0.8.3 was used for the calculation of the Empirical Bayes Geometric Mean (EBGM) as the disproportionality score with its 90% two-sided credibility interval. Drug-event combinations with an EBGM 5% lower limit credibility interval ≥ 2 were considered significant. An EBGM score of 2 indicates that the drug had at least 2 times as many reports for the AE as expected.
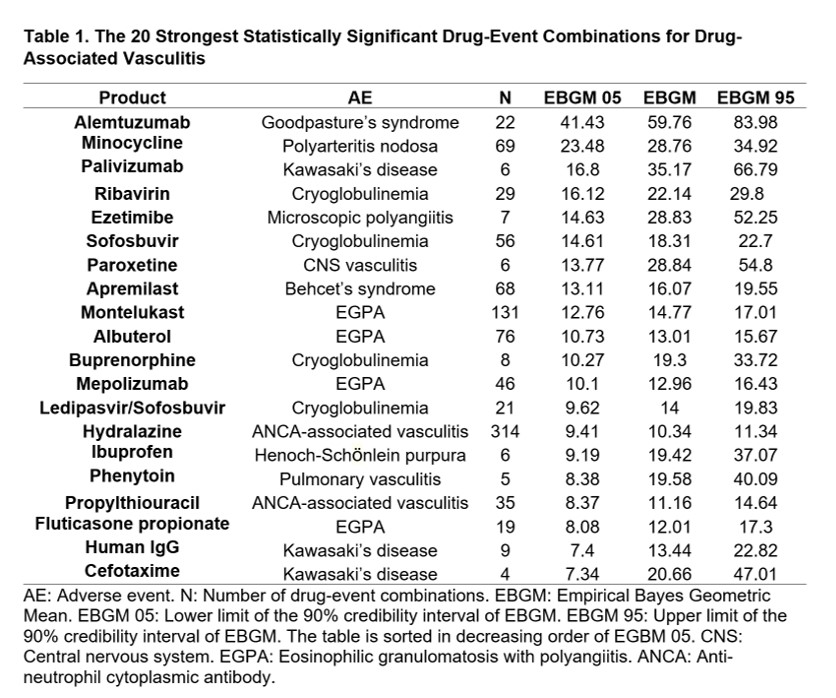
Results: During the study period, we retrieved 10,404 ICSRs reporting 10,931 AEs of vasculitis associated to a medicinal product as the primary suspect for that AE. Patient's mean age was 56.5 years, and 61% of them were female. Of the 25 PTs selected, the generic term "Vasculitis" was the most frequently reported AE (29.1%), followed by "Hypersensitivity vasculitis" (14.9%), "Polymyalgia rheumatica" (7.7%), "Anti-neutrophil cytoplasmic antibody positive vasculitis" (ANCA-associated vasculitis) (6.2%), and "Cutaneous vasculitis" (5.4%). Of the total ICSRs with vasculitis AEs, 90% were expedited because they reported serious, unexpected AEs. Eighty drug-event combinations were disproportionately reported. The strongest association was observed for alemtuzumab and anti-glomerular basement membrane disease (AGBMD), followed by minocycline and polyarteritis nodosa, palivizumab and Kawasaki's disease (KD), then ribavirin and cryoglobulinemia, ezetimibe and microscopic polyangiitis, and so on (Table 1). Some medications were associated with more than one AE, such as infliximab with Takayasu's arteritis (TAK), Behcet's syndrome (BS) and IgA vasculitis; adalimumab with BS and TAK; hydralazine with ANCA-associated vasculitis and renal vasculitis; and rituximab with granulomatosis with polyangiitis and cryoglobulinemia.
Conclusion: Many different vasculitides were reported as AEs in the national database. Some of the medications were associated with the diseases they treat, which raises concern for a possible confounding by indication. In other cases, the events were already known as adverse reactions, for example alemtuzumab and AGBMD or montelukast and eosinophilic granulomatosis with polyangiitis. In other cases, no label or publication references were found, as for palivizumab and KD. These latter cases may raise a concern for potential safety signals that should be investigated.
Disclosures: A. Rodriguez-Pla, None.
Background/Purpose: Vasculitides have been reported as adverse events (AEs) related to a wide variety of medications. We aimed to analyze the vasculitides reported to a national AE spontaneous reporting system from October 2012 through September 2021.
Methods: All spontaneous reports of vasculitis related to any medications from October 1st, 2012, to September 30th, 2021, were retrieved from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database. We performed a descriptive analysis of demographics, medications, and type of vasculitis based on 25 Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) codes. Medications identified as the primary suspect in individual case safety reports (ICSRs) were retrieved by their product active ingredient. RStudio v1.4.1717 was used for general data analysis and the R package openEBGM v0.8.3 was used for the calculation of the Empirical Bayes Geometric Mean (EBGM) as the disproportionality score with its 90% two-sided credibility interval. Drug-event combinations with an EBGM 5% lower limit credibility interval ≥ 2 were considered significant. An EBGM score of 2 indicates that the drug had at least 2 times as many reports for the AE as expected.
Results: During the study period, we retrieved 10,404 ICSRs reporting 10,931 AEs of vasculitis associated to a medicinal product as the primary suspect for that AE. Patient's mean age was 56.5 years, and 61% of them were female. Of the 25 PTs selected, the generic term "Vasculitis" was the most frequently reported AE (29.1%), followed by "Hypersensitivity vasculitis" (14.9%), "Polymyalgia rheumatica" (7.7%), "Anti-neutrophil cytoplasmic antibody positive vasculitis" (ANCA-associated vasculitis) (6.2%), and "Cutaneous vasculitis" (5.4%). Of the total ICSRs with vasculitis AEs, 90% were expedited because they reported serious, unexpected AEs. Eighty drug-event combinations were disproportionately reported. The strongest association was observed for alemtuzumab and anti-glomerular basement membrane disease (AGBMD), followed by minocycline and polyarteritis nodosa, palivizumab and Kawasaki's disease (KD), then ribavirin and cryoglobulinemia, ezetimibe and microscopic polyangiitis, and so on (Table 1). Some medications were associated with more than one AE, such as infliximab with Takayasu's arteritis (TAK), Behcet's syndrome (BS) and IgA vasculitis; adalimumab with BS and TAK; hydralazine with ANCA-associated vasculitis and renal vasculitis; and rituximab with granulomatosis with polyangiitis and cryoglobulinemia.
Conclusion: Many different vasculitides were reported as AEs in the national database. Some of the medications were associated with the diseases they treat, which raises concern for a possible confounding by indication. In other cases, the events were already known as adverse reactions, for example alemtuzumab and AGBMD or montelukast and eosinophilic granulomatosis with polyangiitis. In other cases, no label or publication references were found, as for palivizumab and KD. These latter cases may raise a concern for potential safety signals that should be investigated.

Disclosures: A. Rodriguez-Pla, None.

