Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1426: Year-two Follow-up Results of an Observational Study Conducted in Rheumatoid Arthritis, Ankylosing Spondylitis and Psoriatic Arthritis Patients Treated with Infliximab Biosimilar CT-P13 in a Real-life Setting
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Bruno Fautrel, MD, PhD
Sorbonne University - APHP
Paris, France
Abstract Poster Presenter(s)
Bruno Fautrel1, Maryse Assing2, Nadir Mammar2, Yves Brault2 and Hubert Marotte3, 1Sorbonne University Paris, France and Pierre Louis Institute of Epidemiology and Public Health, Paris, France, Paris, France, 2Pfizer, Paris, France, 3INSERM 1059, Saint-Etienne, France
Background/Purpose: ReFLECT study has been carried out to investigate real life use of CT-P13, the first monoclonal antibody biosimilar to infliximab (IFX) originator.
Methods: ReFLECT is a multicentre, prospective, observational study conducted in France. The primary objectives were to describe the profile of patients (pts) treated with CT-P13 in a real-world setting and the treatment maintenance (described indirectly and expressed as the percentage of pts without treatment failure during the 2 years of follow-up; treatment failure being defined as a permanent discontinuation of CT-P13 due to lack or loss of response and/or intolerance or death). Eligible pts were both IFX naive pts (IFX-N) starting CT-P13 and those who have been switched from infliximab originator to CT-P13 (IFX-S). Final results of 2 years of follow-up of adult pts with rheumatic diseases using descriptive statistical analyses are presented here.
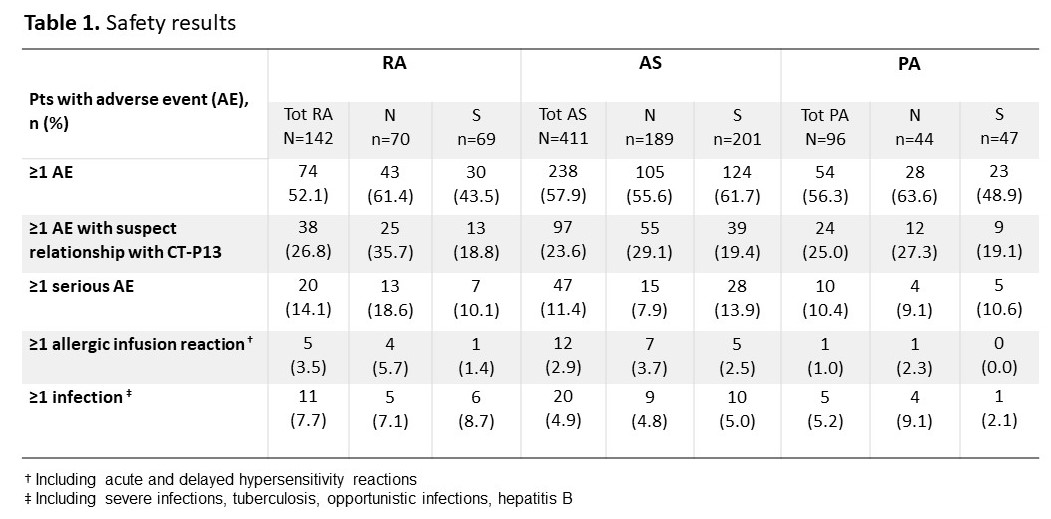
Results: Among the 1,400 adult pts included from October 2016 to April 2019, 751 had an inflammatory bowel disease and 649 had a rheumatic disease including 142 pts with rheumatoid arthritis (RA; 75.4% females; mean age±SD: 61.5±10.9 years old; 70 IFX-N / 69 IFX-S), 411 with ankylosing spondylitis (AS; 41.1% females; 48.1±13.1 years old; 189 IFX-N / 201 IFX-S), and 96 with psoriatic arthritis (PsA; 58.3% females; 53.4±14.1 years old; 44 IFX-N / 47 IFX-S) with a median duration since diagnosis of 13.9, 11.3 and 10.1 years respectively. Nearly 70 to 80% of pts in all indications were already under CT-P13 before inclusion, and 54.2% of RA, 25.3% of AS and 42.7% of PsA pts had a concomitant treatment with methotrexate. At inclusion, 68.3% of RA pts had a DAS28-VS >3.2, 55.9% of AS pts had a BASDAI >4 and 45.7% of PsA pts had a DAS28-VS >3.2 in the IFX-N group compared with 33.3%, 24.7% and 18.9% in the IFX-S group, respectively. From first administration of CT-P13 to 24 months of follow-up in the study, a higher proportion of pts without treatment failure was observed in IFX-S than in IFX-N pts: 65.4% [95%CI: 52.8;81.0] vs 33.3% [22.7;49.1] in RA, 66.5% [58.3;76.0] vs 56.6% [47.6;67.4] in AS and 75.9% [62.2;92.8] vs 72.0% [57.5;90.1] in PsA pts respectively (Kaplan-Meier curves of therapeutic maintenance on Figure 1). Main reason for CT-P13 withdrawing was treatment failure in both naive and switched pts: 38.6% and 18.8% of RA, 23.5% and 18.4% of AS, 22.7% and 13.0% of PsA pts respectively. Withdrawing for intolerance involved 12.9% and 8.7% of RA, 5.3% and 3.0% of AS, 4.5% and 10.9% of PsA in naive and switched pts respectively. Overall, 366 (56.4%) pts reported at least one adverse event (AE). AEs with suspect relationship with CT-P13 were described in 159 (24.5%) pts and serious AEs in 77 (11.9%) patients. Regardless of the indication, AEs and serious AEs were reported similarly in IFX-N (58.1% and 10.6% respectively) and in IFX-S pts (55.8% and 12.6%). Safety results are detailed in Table 1.
Conclusion: Year 2 follow-up data indicate that CT-P13 induced effective therapeutic maintenance in pts with RA, AS and PsA receiving IFX for the first time and as expected, sustained stable results in pts having switched from IFX originator to CT-P13. This real-life study did not highlight any new safety concerns.
.jpg)
Disclosures: B. Fautrel, Pfizer, Novartis, Roche, Sanofi-Aventis, SOBI, UCB; M. Assing, Pfizer; N. Mammar, Pfizer; Y. Brault, Pfizer; H. Marotte, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), CellTrion HealthCare, Fresenius Kabi, HealthCare, Janssen, Eli Lilly, Nordic Pharma, Novartis, Medac, Pfizer, Merck/MSD, Galapagos, UCB.
Background/Purpose: ReFLECT study has been carried out to investigate real life use of CT-P13, the first monoclonal antibody biosimilar to infliximab (IFX) originator.
Methods: ReFLECT is a multicentre, prospective, observational study conducted in France. The primary objectives were to describe the profile of patients (pts) treated with CT-P13 in a real-world setting and the treatment maintenance (described indirectly and expressed as the percentage of pts without treatment failure during the 2 years of follow-up; treatment failure being defined as a permanent discontinuation of CT-P13 due to lack or loss of response and/or intolerance or death). Eligible pts were both IFX naive pts (IFX-N) starting CT-P13 and those who have been switched from infliximab originator to CT-P13 (IFX-S). Final results of 2 years of follow-up of adult pts with rheumatic diseases using descriptive statistical analyses are presented here.
Results: Among the 1,400 adult pts included from October 2016 to April 2019, 751 had an inflammatory bowel disease and 649 had a rheumatic disease including 142 pts with rheumatoid arthritis (RA; 75.4% females; mean age±SD: 61.5±10.9 years old; 70 IFX-N / 69 IFX-S), 411 with ankylosing spondylitis (AS; 41.1% females; 48.1±13.1 years old; 189 IFX-N / 201 IFX-S), and 96 with psoriatic arthritis (PsA; 58.3% females; 53.4±14.1 years old; 44 IFX-N / 47 IFX-S) with a median duration since diagnosis of 13.9, 11.3 and 10.1 years respectively. Nearly 70 to 80% of pts in all indications were already under CT-P13 before inclusion, and 54.2% of RA, 25.3% of AS and 42.7% of PsA pts had a concomitant treatment with methotrexate. At inclusion, 68.3% of RA pts had a DAS28-VS >3.2, 55.9% of AS pts had a BASDAI >4 and 45.7% of PsA pts had a DAS28-VS >3.2 in the IFX-N group compared with 33.3%, 24.7% and 18.9% in the IFX-S group, respectively. From first administration of CT-P13 to 24 months of follow-up in the study, a higher proportion of pts without treatment failure was observed in IFX-S than in IFX-N pts: 65.4% [95%CI: 52.8;81.0] vs 33.3% [22.7;49.1] in RA, 66.5% [58.3;76.0] vs 56.6% [47.6;67.4] in AS and 75.9% [62.2;92.8] vs 72.0% [57.5;90.1] in PsA pts respectively (Kaplan-Meier curves of therapeutic maintenance on Figure 1). Main reason for CT-P13 withdrawing was treatment failure in both naive and switched pts: 38.6% and 18.8% of RA, 23.5% and 18.4% of AS, 22.7% and 13.0% of PsA pts respectively. Withdrawing for intolerance involved 12.9% and 8.7% of RA, 5.3% and 3.0% of AS, 4.5% and 10.9% of PsA in naive and switched pts respectively. Overall, 366 (56.4%) pts reported at least one adverse event (AE). AEs with suspect relationship with CT-P13 were described in 159 (24.5%) pts and serious AEs in 77 (11.9%) patients. Regardless of the indication, AEs and serious AEs were reported similarly in IFX-N (58.1% and 10.6% respectively) and in IFX-S pts (55.8% and 12.6%). Safety results are detailed in Table 1.
Conclusion: Year 2 follow-up data indicate that CT-P13 induced effective therapeutic maintenance in pts with RA, AS and PsA receiving IFX for the first time and as expected, sustained stable results in pts having switched from IFX originator to CT-P13. This real-life study did not highlight any new safety concerns.
.jpg)

Disclosures: B. Fautrel, Pfizer, Novartis, Roche, Sanofi-Aventis, SOBI, UCB; M. Assing, Pfizer; N. Mammar, Pfizer; Y. Brault, Pfizer; H. Marotte, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), CellTrion HealthCare, Fresenius Kabi, HealthCare, Janssen, Eli Lilly, Nordic Pharma, Novartis, Medac, Pfizer, Merck/MSD, Galapagos, UCB.

