Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0416: Efficacy and Safety of Biological DMARDs: A Systematic Literature Review Informing the 2022 Update of the ASAS-EULAR Recommendations for the Management of Axial Spondyloarthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Augusta Ortolan, MD
University of Padova
Padova, Padua, Italy
Abstract Poster Presenter(s)
Casper Webers1, Augusta Ortolan2, Alexandre Sepriano3, Louise Falzon4, Xenofon Baraliakos5, Robert Landewé6, Sofia Ramiro7, Désirée van der Heijde8 and Elena Nikiphorou9, 1Maastricht University Medical Centre, Maastricht, Netherlands, 2University of Padova/Leiden University Medical Center, Padova, Italy, 3Leiden University Medical Centre, Portela Loures, Portugal, 4Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, United Kingdom, 5Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 6Amsterdam University Medical Center, Meerssen, Netherlands, 7Leiden University Medical Center, Leiden, Netherlands, 8Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Netherlands, 9Leiden University Medical Center & King's College London, London, United Kingdom
Background/Purpose: Since the 2016 update of the management recommendations for axial spondyloarthritis (axSpA), new evidence has emerged on the efficacy and safety of biological disease-modifying antirheumatic drugs (bDMARDs) in axSpA. Our aim was to summarize this new evidence to inform the taskforce of the 2022 update of the ASAS-EULAR recommendations for the management of axSpA on this topic.
Methods: Systematic literature review of studies published between 2016-2021 on the efficacy and safety of bDMARDs in axSpA (including r-axSpA and nr-axSpA) (PROSPERO registration CRD42021257588). Eligible study designs included randomised controlled trials (RCTs), strategy trials and observational studies (the latter only for safety and extra-musculoskeletal manifestations). All relevant efficacy/safety outcomes were included. Risk ratios (RR) were used as effect measure for efficacy.
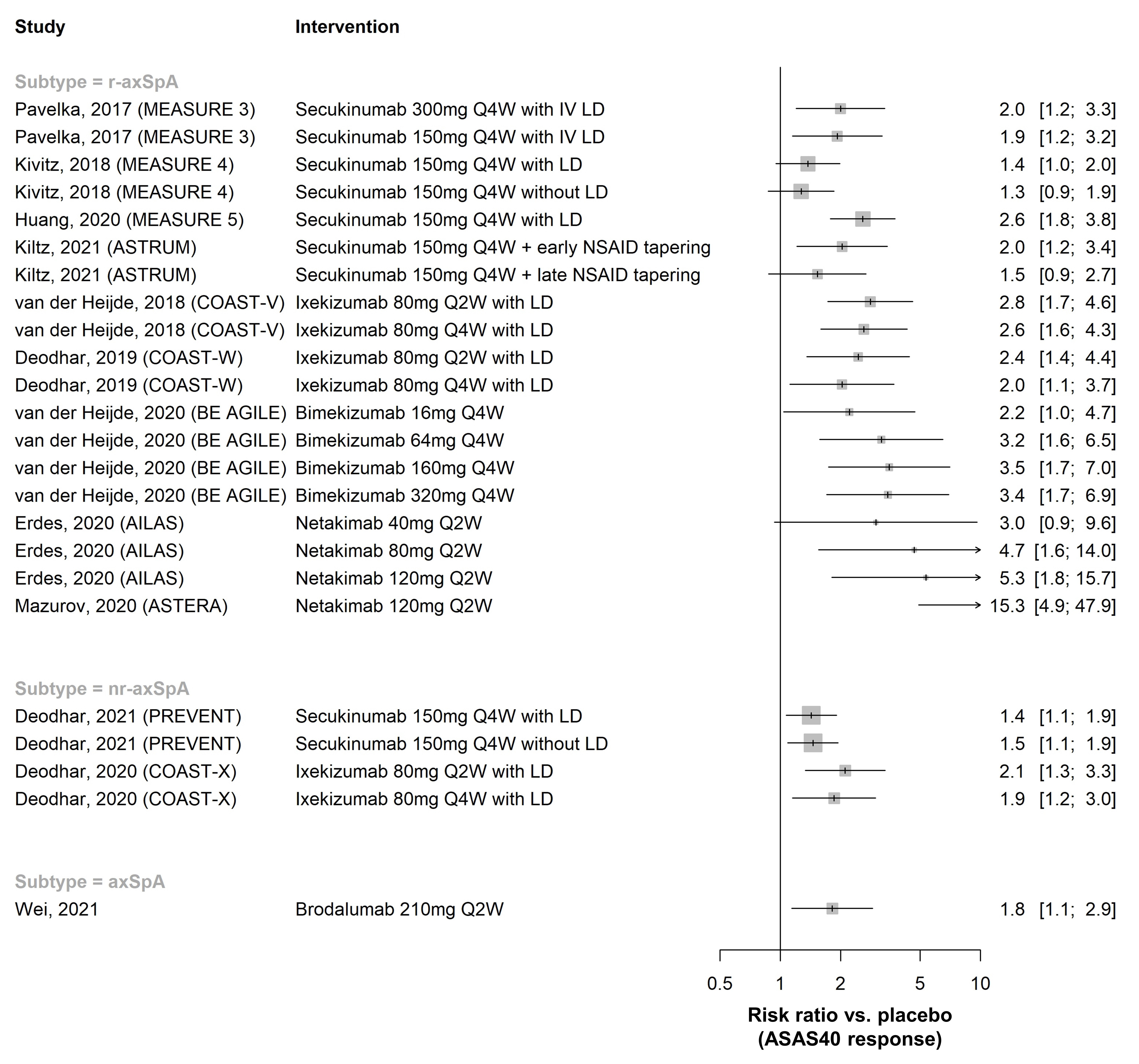
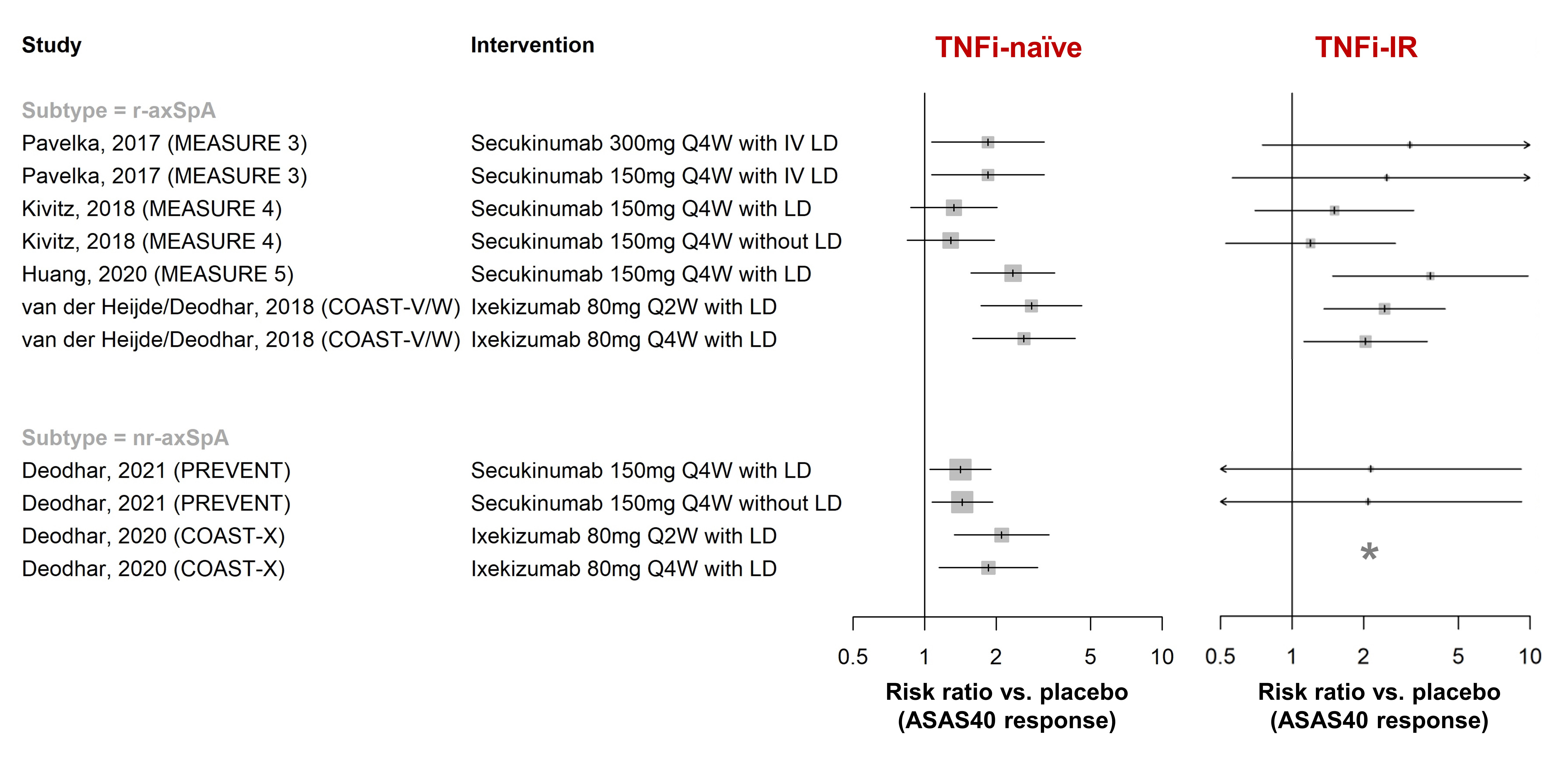
Results: In total, 148 publications (91 full-text articles and 57 conference abstracts) were included. Efficacy of golimumab and certolizumab was confirmed in r-axSpA and nr-axSpA, respectively. Equivalence between adalimumab/etanercept/infliximab biosimilars and originators was demonstrated (n=1 each). Fourteen placebo-controlled RCTs investigated efficacy of interleukin-17 inhibitors (IL-17i: secukinumab [n=7], ixekizumab [n=3], netakimab [n=2], brodalumab [n=1], bimekizumab [n=1]) and found clinically relevant effects (RR versus placebo to achieve ASAS40 response 1.3-15.3 [r-axSpA, n=9], 1.4-2.1 [nr-axSpA, n=2]; Figure 1). Efficacy of secukinumab/ixekizumab was demonstrated both in TNFi-naïve and TNFi-inadequate responders, with response rates being higher in TNFi-naïve patients (Figure 2). Trials of IL-23 and IL-12/23 inhibitors (IL-23i: risankizumab [n=1]; IL-12/23i: ustekinumab [n=3]) failed to show relevant benefits compared to placebo and were stopped. Discontinuation of TNFi (n=3) and IL-17i (n=1) resulted in high rates of flare (45-80% within 48 weeks), while tapering of TNFi by spacing was non-inferior to standard-dose treatment in maintaining disease control (n=2). The first axSpA treat-to-target trial did not meet its primary endpoint (ASAS-HI 30% improvement) but showed improvements in secondary outcomes (ASAS20/40). No new risks with TNFi were found in observational studies (no data yet for IL-17i in observational studies). Secukinumab and etanercept were associated with increased risk of uveitis compared to monoclonal TNFi in one and two observational studies, respectively.
Conclusion: New evidence supports the efficacy and safety of TNFi (originators/biosimilars) and IL-17i in r-axSpA and nr-axSpA, while IL-12/23i and IL-23i failed to show relevant effects. This systematic review provides important new insights into the management of patients with bDMARDs, across the entire spectrum of axSpA.
Disclosures: C. Webers, None; A. Ortolan, None; A. Sepriano, UCB, Novartis; L. Falzon, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.
Background/Purpose: Since the 2016 update of the management recommendations for axial spondyloarthritis (axSpA), new evidence has emerged on the efficacy and safety of biological disease-modifying antirheumatic drugs (bDMARDs) in axSpA. Our aim was to summarize this new evidence to inform the taskforce of the 2022 update of the ASAS-EULAR recommendations for the management of axSpA on this topic.
Methods: Systematic literature review of studies published between 2016-2021 on the efficacy and safety of bDMARDs in axSpA (including r-axSpA and nr-axSpA) (PROSPERO registration CRD42021257588). Eligible study designs included randomised controlled trials (RCTs), strategy trials and observational studies (the latter only for safety and extra-musculoskeletal manifestations). All relevant efficacy/safety outcomes were included. Risk ratios (RR) were used as effect measure for efficacy.
Results: In total, 148 publications (91 full-text articles and 57 conference abstracts) were included. Efficacy of golimumab and certolizumab was confirmed in r-axSpA and nr-axSpA, respectively. Equivalence between adalimumab/etanercept/infliximab biosimilars and originators was demonstrated (n=1 each). Fourteen placebo-controlled RCTs investigated efficacy of interleukin-17 inhibitors (IL-17i: secukinumab [n=7], ixekizumab [n=3], netakimab [n=2], brodalumab [n=1], bimekizumab [n=1]) and found clinically relevant effects (RR versus placebo to achieve ASAS40 response 1.3-15.3 [r-axSpA, n=9], 1.4-2.1 [nr-axSpA, n=2]; Figure 1). Efficacy of secukinumab/ixekizumab was demonstrated both in TNFi-naïve and TNFi-inadequate responders, with response rates being higher in TNFi-naïve patients (Figure 2). Trials of IL-23 and IL-12/23 inhibitors (IL-23i: risankizumab [n=1]; IL-12/23i: ustekinumab [n=3]) failed to show relevant benefits compared to placebo and were stopped. Discontinuation of TNFi (n=3) and IL-17i (n=1) resulted in high rates of flare (45-80% within 48 weeks), while tapering of TNFi by spacing was non-inferior to standard-dose treatment in maintaining disease control (n=2). The first axSpA treat-to-target trial did not meet its primary endpoint (ASAS-HI 30% improvement) but showed improvements in secondary outcomes (ASAS20/40). No new risks with TNFi were found in observational studies (no data yet for IL-17i in observational studies). Secukinumab and etanercept were associated with increased risk of uveitis compared to monoclonal TNFi in one and two observational studies, respectively.
Conclusion: New evidence supports the efficacy and safety of TNFi (originators/biosimilars) and IL-17i in r-axSpA and nr-axSpA, while IL-12/23i and IL-23i failed to show relevant effects. This systematic review provides important new insights into the management of patients with bDMARDs, across the entire spectrum of axSpA.

Figure 1. ASAS40 response in placebo-controlled RCTs of IL-17 inhibitors, by axSpA subtype. Risk ratios >1 favour the intervention. Only RCTs that reported ASAS40 response rates are shown (12 out of 14 included). Numbers on the right are RR with 95% confidence interval. LD = loading dose.

Figure 2. ASAS40 response in placebo-controlled RCTs of IL-17 inhibitors, by prior TNFi history and axSpA subtype. Risk ratios >1 favour the intervention. With the exception of MEASURE 5 and PREVENT, all studies either stratified randomisation for prior TNFi treatment, or only included TNFi-naïve or TNFi-IR patients (not both). *COAST-X only included TNFi-naïve patients. LD = loading dose, TNFi-IR = inadequate responder to prior TNFi treatment.
Disclosures: C. Webers, None; A. Ortolan, None; A. Sepriano, UCB, Novartis; L. Falzon, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.

