Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0253: Evaluation of Patients with Rheumatoid Arthritis in Teleconsultation During the First Wave of the COVID-19 Pandemic
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jérôme Avouac, MD, PhD
Université de Paris Cité
Paris, France
Abstract Poster Presenter(s)
Jérôme Avouac1, Anna Molto2, Camelia Frantz3, sarah wanono3, elise descamps4, olivier fogel4, Alice Combier5, lucile poiroux5, Corinne MIceli6 and Yannick Allanore7, 1University of Paris, Paris, France, 2Rheumatology Department, Hôpital Cochin,Assistance Publique- Hôpitaux de Paris, Paris, France, 3Hôpital Cochin, AP-HP Centre - Université Paris Cité, Paris, France, 4Hôpital Cochin, AP-HP Centre - Université de Paris Cité, Paris, France, 5Hôpital Cochin, AP-HP.Centre - Université Paris Cité, Paris, France, 6APHP, Paris, France, 7Department of Rheumatology A, Descartes University, APHP, Cochin Hospital, Paris, France, Paris, France
Background/Purpose: The sudden emergence of SARS-CoV-2 onto the world stage has accelerated a major change in the management of patients with chronic rheumatic diseases and has catalyzed the rapid emergence of telemedicine. Our aim was to describe which parameters were collected by rheumatologists to monitor patients with rheumatoid arthritis (RA) during teleconsultation and identify which ones have more impact on clinician intervention.
Methods: Retrospective monocentric routine care cross-sectional study including RA patients seen in teleconsultation between March and September 2020. Available parameters assessing disease status were collected in teleconsultation files. Clinician intervention was defined by treatment escalation and/or the need for a rapid face-to-face consultation or day hospitalization.
Results: 143 RA patients were included (117 females, mean age of 58±16 years, mean disease duration of 14±11 years). The presence or absence of patient self-reported RA flares was mentioned in all medical files, followed by the presence and/or the number of tender joints (76%), the duration of morning stiffness (66%), the number of pain-related nocturnal awakenings (66%) and the CRP value (54%).
Teleconsultation led to a clinician intervention in 22/143 patients (14%), representing 51% of patients with self-reported flares (22/43 patients). Patients requesting clinical intervention had a shorter disease duration (10±10 years vs. 15±11 years, p=0.049), a lower frequency of erosions (19% vs. 60%, p< 0.001), a more active disease, and a higher likelihood of corticosteroid therapy (73% vs. 42%, p=0.007).Therapeutic escalation was necessary in 13 patients: introduction or dose increase of corticosteroids in 8 patients, introduction or dose increase of methotrexate in 4 patients and introduction of hydroxychloroquine in 1 patient. Face-to-face consultation or day hospitalization were organized for 10 patients. Active disease was confirmed during this next face-to-face visit in 9 patients, with DAS28 ranging from 3.35 to 5.62, leading to therapeutic modification. The 133 other patients were seen in face-to-face consultation 6±2 months after the teleconsultation. No DMARD modification was recorded during this next face-to-face consultation.
The following variables were associated with clinician intervention during the teleconsultation in univariate analysis: patient self-reported RA flares since the last visit (p< 0.001), CRP >10 mg/mL (p=0.003) and a morning stiffness > 30 minutes (p< 0.001). Multivariate analysis confirmed RA flares (Odds Ratio, OR: 15.6 95% CI 3.37-68.28) and CRP values >10 mg/L (OR: 3.32, 95% CI % 1.12-13.27) as the variables independently associated with clinician intervention (Table 1).
Conclusion: Our study showed the reliability of clinician intervention in teleconsultation and identified patient reported RA flares and increased CRP values as 2 red flags, independently associated with therapeutic modification and/or the need for a rapid face-to-face consultation. These indicators may help clinician's decision making in teleconsultation and need to be confirmed in independent cohorts.
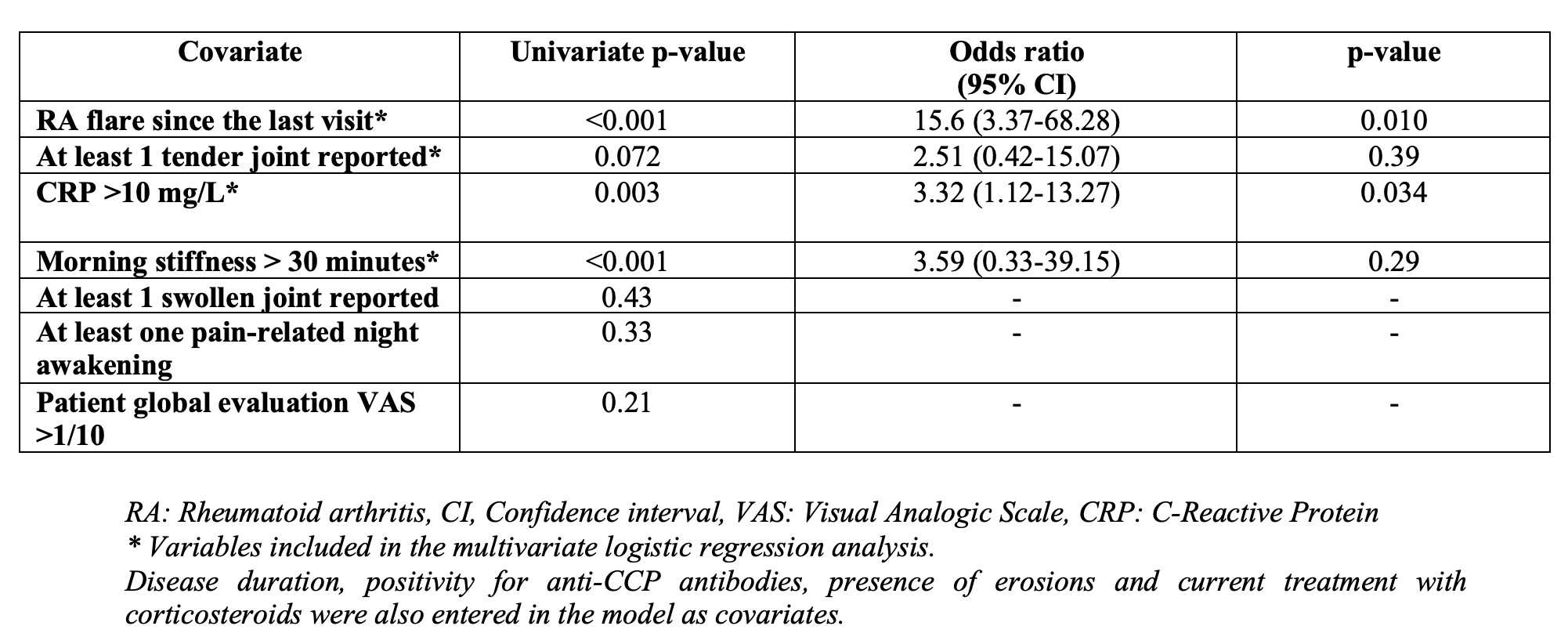 Table 1: Logistic regression analysis including clinician intervention as the dependent variable
Table 1: Logistic regression analysis including clinician intervention as the dependent variable
Disclosures: J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; A. Molto, Abbvie, UCB, Novartis, Gilead, Pfizer, Lilly, Jannsen; C. Frantz, None; s. wanono, None; e. descamps, None; o. fogel, None; A. Combier, None; l. poiroux, None; C. MIceli, None; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca.
Background/Purpose: The sudden emergence of SARS-CoV-2 onto the world stage has accelerated a major change in the management of patients with chronic rheumatic diseases and has catalyzed the rapid emergence of telemedicine. Our aim was to describe which parameters were collected by rheumatologists to monitor patients with rheumatoid arthritis (RA) during teleconsultation and identify which ones have more impact on clinician intervention.
Methods: Retrospective monocentric routine care cross-sectional study including RA patients seen in teleconsultation between March and September 2020. Available parameters assessing disease status were collected in teleconsultation files. Clinician intervention was defined by treatment escalation and/or the need for a rapid face-to-face consultation or day hospitalization.
Results: 143 RA patients were included (117 females, mean age of 58±16 years, mean disease duration of 14±11 years). The presence or absence of patient self-reported RA flares was mentioned in all medical files, followed by the presence and/or the number of tender joints (76%), the duration of morning stiffness (66%), the number of pain-related nocturnal awakenings (66%) and the CRP value (54%).
Teleconsultation led to a clinician intervention in 22/143 patients (14%), representing 51% of patients with self-reported flares (22/43 patients). Patients requesting clinical intervention had a shorter disease duration (10±10 years vs. 15±11 years, p=0.049), a lower frequency of erosions (19% vs. 60%, p< 0.001), a more active disease, and a higher likelihood of corticosteroid therapy (73% vs. 42%, p=0.007).Therapeutic escalation was necessary in 13 patients: introduction or dose increase of corticosteroids in 8 patients, introduction or dose increase of methotrexate in 4 patients and introduction of hydroxychloroquine in 1 patient. Face-to-face consultation or day hospitalization were organized for 10 patients. Active disease was confirmed during this next face-to-face visit in 9 patients, with DAS28 ranging from 3.35 to 5.62, leading to therapeutic modification. The 133 other patients were seen in face-to-face consultation 6±2 months after the teleconsultation. No DMARD modification was recorded during this next face-to-face consultation.
The following variables were associated with clinician intervention during the teleconsultation in univariate analysis: patient self-reported RA flares since the last visit (p< 0.001), CRP >10 mg/mL (p=0.003) and a morning stiffness > 30 minutes (p< 0.001). Multivariate analysis confirmed RA flares (Odds Ratio, OR: 15.6 95% CI 3.37-68.28) and CRP values >10 mg/L (OR: 3.32, 95% CI % 1.12-13.27) as the variables independently associated with clinician intervention (Table 1).
Conclusion: Our study showed the reliability of clinician intervention in teleconsultation and identified patient reported RA flares and increased CRP values as 2 red flags, independently associated with therapeutic modification and/or the need for a rapid face-to-face consultation. These indicators may help clinician's decision making in teleconsultation and need to be confirmed in independent cohorts.
 Table 1: Logistic regression analysis including clinician intervention as the dependent variable
Table 1: Logistic regression analysis including clinician intervention as the dependent variableDisclosures: J. Avouac, Galapagos, AbbVie, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Fresenius-Kabi, Sanofi, Sandoz, Nordic Pharma, Biogen, Medac, Janssen, Roche-Chugai; A. Molto, Abbvie, UCB, Novartis, Gilead, Pfizer, Lilly, Jannsen; C. Frantz, None; s. wanono, None; e. descamps, None; o. fogel, None; A. Combier, None; l. poiroux, None; C. MIceli, None; Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca.

