Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0290: Evaluation of Treatment Discontinuation Due to Adverse Events, and the Effect of Cardiovascular Risk Factors or Type of JAK-inhibitors: An International Collaboration of Registries of Rheumatoid Arthritis Patients (the "JAK-pot" Study)
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- KL
Kim Lauper, MD
Geneva University Hospitals
Genève, Switzerland
Abstract Poster Presenter(s)
Kim Lauper1, Romain Aymon2, Denis Mongin2, Sytske Anne Bergstra3, Denis Choquette4, Catalin Codreanu5, Ori Elkayam6, Kimme Hyrich7, Florenzo Iannone8, Nevsun Inanc9, Lianne Kearsley-Fleet10, Tore K. kvien11, Eirik Kristianslund12, Burkhard Leeb13, Galina Lukina14, Dan Nordstrom15, Karel Pavelka16, Manuel Pombo-Suarez17, Ziga Rotar18, Maria José Santos19, Delphine Courvoisier20 and Axel Finckh21, 1Geneva University Hospitals, Genéve, Switzerland, 2Geneva University Hospitals, Geneva, Switzerland, 3LUMC, Leiden, Netherlands, 4Centre Hospitalier de l'Université de Montréal, Montréal, QC, Canada, 5Center for Rheumatic Diseases, Bucharest, Romania, 6Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 7The University of Manchester, Manchester, United Kingdom, 8School of Medicine University of Bari, Bari, Italy, 9Marmara University School of Medicine, Division of Rheumatology, Istanbul, Turkey, 10Centre for Epidemiology Versus Arthritis, University of Manchester, Manchester, United Kingdom, 11Diakonhjemmet Hospital, Oslo, Norway, 12Diakonhjemmet Hospital, Division of Rheumatology and Research, Oslo, Norway, 13Bioreg, Stockerau, Austria, 14Federal state budgetary scientific institution �Research Institute of rheumatology named after V. A. Nasonova�, Moscow, Russia, 15Helsinki University Hospital, Helsinki, Finland, 16Institute of Rheumatology, Department of Rheumatology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, Praha, Czech Republic, 17Hospital Clinico Universitario, Santiago de Compostela, Spain, 18University Medical Centre Ljubljana, Ljubljana, Slovenia, 19Hospital Garcia de Orta, Almada, Charneca da Caparica, Portugal, 20University Hospitals of Geneva, Geneva, Switzerland, 21Geneva University Hospital, Geneve - Vesenaz, Switzerland
Background/Purpose: The "ORAL Surveillance" study1 suggests an increased risk of serious adverse events (AEs) with tofacitinib, a JAK-inhibitor (JAKi), compared to TNF-inhibitors (TNFi). Currently, there is limited real-world evidence for the tolerability and safety of JAKi. This study aims to assess the safety of JAKi compared to other biologics in a real-world population, evaluating treatment discontinuation for AEs and the effect of cardiovascular risk factors and type of JAKi on this outcome.
Methods: We pooled the data from 16 national RA registries from across Europe, Québec (Canada), Turkey, and Israel. We compared treatment discontinuation due to AEs by treatment groups, JAKi vs TNFi, and JAKi vs bDMARDs with other modes of action (OMA), as an overall measure of tolerability and safety of JAKi. We included only treatment courses initiated during a period when all treatment options were available, to limit potential selection biases. We used standard descriptive statistics for baseline characteristics. We plotted unadjusted cumulative incidence, then we used a cause-specific Cox model accounting for competing risks of stopping for ineffectiveness or other reasons, to obtain association between covariates and the instantaneous hazard rate for AEs. Variables listed in Table 1 were used for adjustment in the fully-adjusted cause-specific Cox model. A subgroup analysis examined only patients with age >50 years and at least one cardiovascular risk factor, as in ORAL-surveillance (RCT-duplicate cohort). We also investigated whether results differed by type of JAKi (tofacitinib vs others).
Results: 43,748 treatment courses were included in the analysis (Table 1). In the JAKi group, 50% of patients were treated with tofacitinib, and 44% with baricitinib, with only a few patients treated with upadacitinib or filgotinib. We observed higher treatment discontinuation due to AEs with OMA compared to JAKi, and similar rates between JAKi and TNFi (Figure 1). The fully adjusted hazard rate of treatment discontinuation for AEs was also similar for TNFi vs JAKi (aHR 1.11, 95%CI 0.98 to 1.25), but higher for OMA vs JAKi (aHR 1.23, 95% CI 1.09 to 1.40, Figure 2). The rate of treatment discontinuation for AEs was higher in women than men (aHR 1.36, 95%CI 1.22 to 1.51). The results in the RCT-duplicate subpopulation cohort (35% of all treatment, N=15,541) were similar than in the full cohort, with higher treatment discontinuation for OMA vs JAKi (aHR 1.38, 95%CI 1.14-1.66) and similar for TNFi vs JAKi (aHR 1.12, 95%CI 0.94 to 1.34) although there was overall more treatment discontinuation for AEs. These ratios were also similar for OMA and TNFi vs tofacitinib or vs other JAKis.
Conclusion: After adjusting for potential confounders, the rate of treatment discontinuation for AEs was comparable between JAKi and TNFi, but higher for OMA vs JAKi. We found no differences in these ratios by type of JAKi (tofacitinib or others, mostly baricitinib) or in the RCT-duplicate cohort compared to the overall population, although treatment discontinuation for AEs was higher overall in this subgroup.
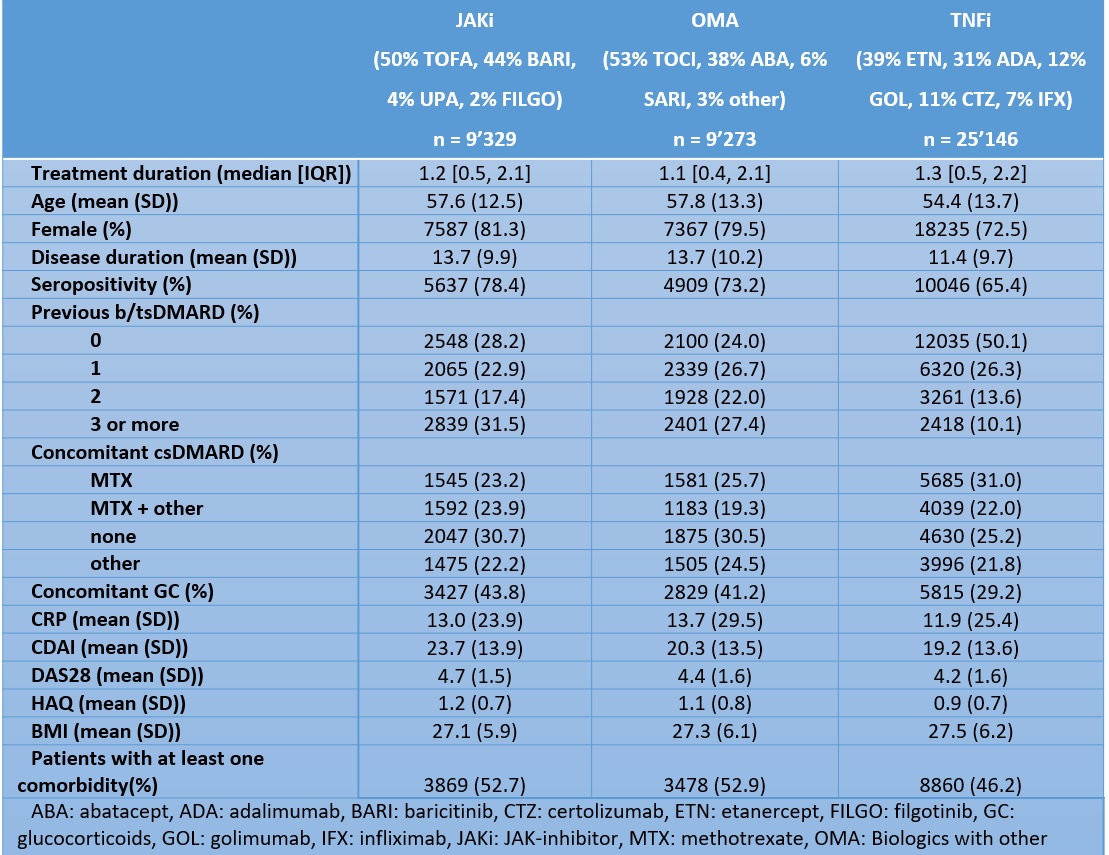 Table 1: Baseline characteristics of the population by treatment groups: Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA)
Table 1: Baseline characteristics of the population by treatment groups: Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA)
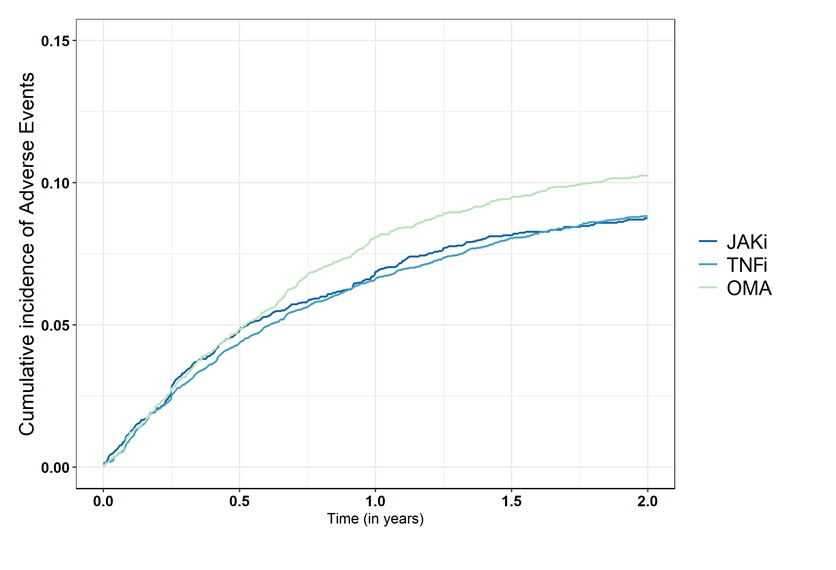 Figure 1: Comparison of the crude cumulative incidence of treatment discontinuation for adverse events among the Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA) groups.
Figure 1: Comparison of the crude cumulative incidence of treatment discontinuation for adverse events among the Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA) groups.
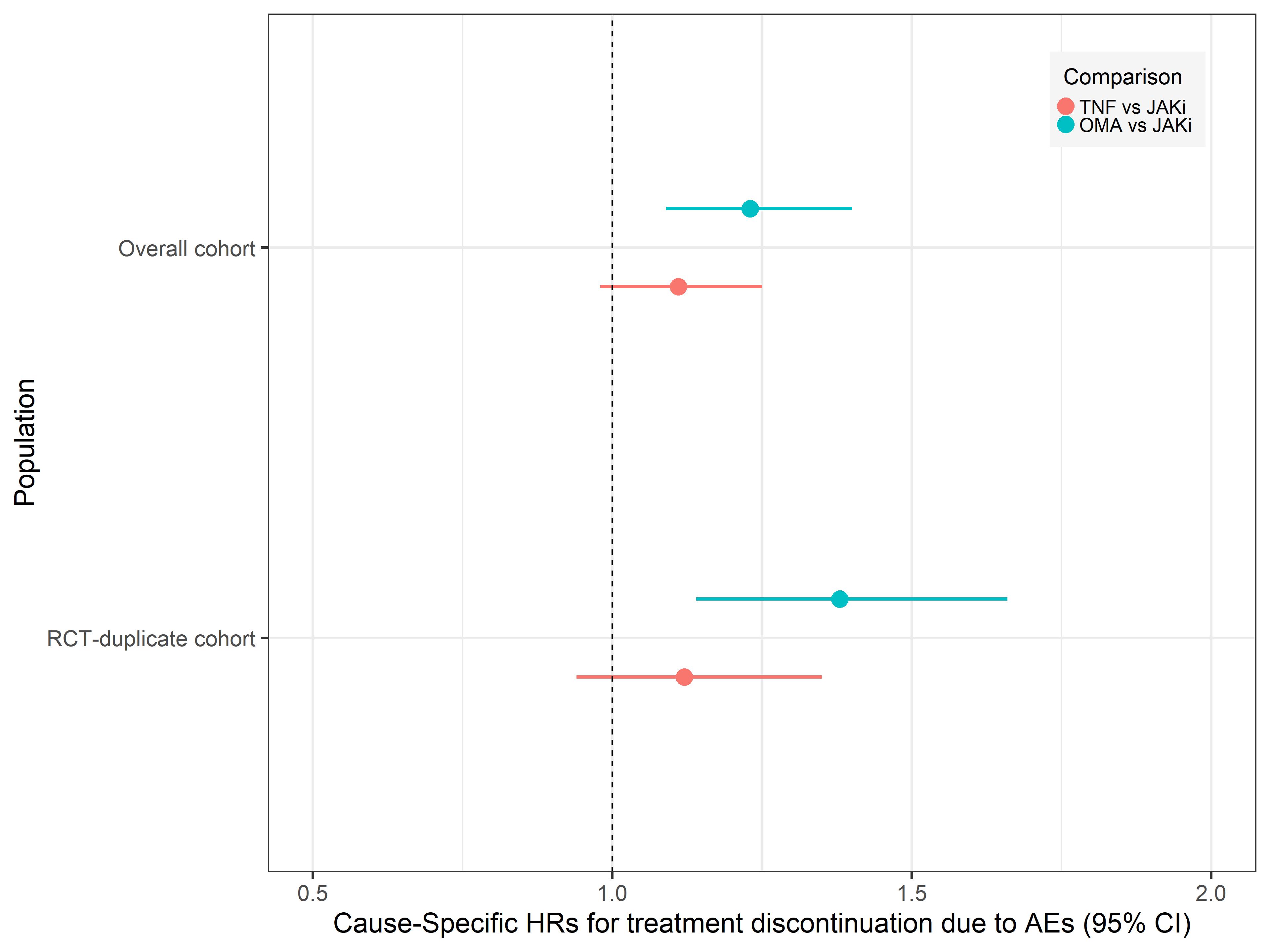 Figure 2: Adjusted hazards ratios of discontinuation for adverse events for TNFi-inhibitors (TNFi) vs Janus kinase inhibitors (JAK) and biologics with other modes of action (OMA) vs JAKi
Figure 2: Adjusted hazards ratios of discontinuation for adverse events for TNFi-inhibitors (TNFi) vs Janus kinase inhibitors (JAK) and biologics with other modes of action (OMA) vs JAKi
Disclosures: K. Lauper, Pfizer, Viatris, Celltrion; R. Aymon, None; D. Mongin, None; S. Bergstra, None; D. Choquette, AbbVie/Abbott, Amgen, Eli Lilly, Fresenius Kabi, Novartis, Pfizer, Sandoz, Tavapharm; C. Codreanu, AbbVie/Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Ewopharma, Eli Lilly, Novartis, Pfizer; O. Elkayam, Pfizer, Eli Lilly, AbbVie/Abbott, Novartis, Janssen, Boehringer-Ingelheim; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); F. Iannone, None; N. Inanc, AbbVie/Abbott, Eli Lilly, Merck/MSD, novartis, Pfizer, Roche, Amgen, Celltrion, UCB; L. Kearsley-Fleet, None; T. kvien, Pfizer, AbbVie/Abbott, Eli Lilly, galapagos; E. Kristianslund, None; B. Leeb, Sandoz, AbbVie/Abbott, Eli Lilly, Roche, Grunenthal, Biogen, Celgene; G. Lukina, AbbVie/Abbott, Eli Lilly, Merck/MSD, Roche, Pfizer; D. Nordstrom, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, Novartis, Pfizer, Roche, UCB; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; M. Pombo-Suarez, Janssen, Eli Lilly, Merck/MSD, Novartis, sanofi; Z. Rotar, Pfizer, AbbVie/Abbott, Eli Lilly; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; D. Courvoisier, None; A. Finckh, Pfizer, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, AbbVie/Abbott, galapagos, mylan, UCB, Viatris.
Background/Purpose: The "ORAL Surveillance" study1 suggests an increased risk of serious adverse events (AEs) with tofacitinib, a JAK-inhibitor (JAKi), compared to TNF-inhibitors (TNFi). Currently, there is limited real-world evidence for the tolerability and safety of JAKi. This study aims to assess the safety of JAKi compared to other biologics in a real-world population, evaluating treatment discontinuation for AEs and the effect of cardiovascular risk factors and type of JAKi on this outcome.
Methods: We pooled the data from 16 national RA registries from across Europe, Québec (Canada), Turkey, and Israel. We compared treatment discontinuation due to AEs by treatment groups, JAKi vs TNFi, and JAKi vs bDMARDs with other modes of action (OMA), as an overall measure of tolerability and safety of JAKi. We included only treatment courses initiated during a period when all treatment options were available, to limit potential selection biases. We used standard descriptive statistics for baseline characteristics. We plotted unadjusted cumulative incidence, then we used a cause-specific Cox model accounting for competing risks of stopping for ineffectiveness or other reasons, to obtain association between covariates and the instantaneous hazard rate for AEs. Variables listed in Table 1 were used for adjustment in the fully-adjusted cause-specific Cox model. A subgroup analysis examined only patients with age >50 years and at least one cardiovascular risk factor, as in ORAL-surveillance (RCT-duplicate cohort). We also investigated whether results differed by type of JAKi (tofacitinib vs others).
Results: 43,748 treatment courses were included in the analysis (Table 1). In the JAKi group, 50% of patients were treated with tofacitinib, and 44% with baricitinib, with only a few patients treated with upadacitinib or filgotinib. We observed higher treatment discontinuation due to AEs with OMA compared to JAKi, and similar rates between JAKi and TNFi (Figure 1). The fully adjusted hazard rate of treatment discontinuation for AEs was also similar for TNFi vs JAKi (aHR 1.11, 95%CI 0.98 to 1.25), but higher for OMA vs JAKi (aHR 1.23, 95% CI 1.09 to 1.40, Figure 2). The rate of treatment discontinuation for AEs was higher in women than men (aHR 1.36, 95%CI 1.22 to 1.51). The results in the RCT-duplicate subpopulation cohort (35% of all treatment, N=15,541) were similar than in the full cohort, with higher treatment discontinuation for OMA vs JAKi (aHR 1.38, 95%CI 1.14-1.66) and similar for TNFi vs JAKi (aHR 1.12, 95%CI 0.94 to 1.34) although there was overall more treatment discontinuation for AEs. These ratios were also similar for OMA and TNFi vs tofacitinib or vs other JAKis.
Conclusion: After adjusting for potential confounders, the rate of treatment discontinuation for AEs was comparable between JAKi and TNFi, but higher for OMA vs JAKi. We found no differences in these ratios by type of JAKi (tofacitinib or others, mostly baricitinib) or in the RCT-duplicate cohort compared to the overall population, although treatment discontinuation for AEs was higher overall in this subgroup.
Ref: 1. Ytterberg SR et al. N Engl J Med. 2022 Jan 27;386(4):316-326. doi: 10.1056/NEJMoa2109927
 Table 1: Baseline characteristics of the population by treatment groups: Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA)
Table 1: Baseline characteristics of the population by treatment groups: Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA)  Figure 1: Comparison of the crude cumulative incidence of treatment discontinuation for adverse events among the Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA) groups.
Figure 1: Comparison of the crude cumulative incidence of treatment discontinuation for adverse events among the Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and biologics with other modes of action (OMA) groups.  Figure 2: Adjusted hazards ratios of discontinuation for adverse events for TNFi-inhibitors (TNFi) vs Janus kinase inhibitors (JAK) and biologics with other modes of action (OMA) vs JAKi
Figure 2: Adjusted hazards ratios of discontinuation for adverse events for TNFi-inhibitors (TNFi) vs Janus kinase inhibitors (JAK) and biologics with other modes of action (OMA) vs JAKiDisclosures: K. Lauper, Pfizer, Viatris, Celltrion; R. Aymon, None; D. Mongin, None; S. Bergstra, None; D. Choquette, AbbVie/Abbott, Amgen, Eli Lilly, Fresenius Kabi, Novartis, Pfizer, Sandoz, Tavapharm; C. Codreanu, AbbVie/Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Ewopharma, Eli Lilly, Novartis, Pfizer; O. Elkayam, Pfizer, Eli Lilly, AbbVie/Abbott, Novartis, Janssen, Boehringer-Ingelheim; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); F. Iannone, None; N. Inanc, AbbVie/Abbott, Eli Lilly, Merck/MSD, novartis, Pfizer, Roche, Amgen, Celltrion, UCB; L. Kearsley-Fleet, None; T. kvien, Pfizer, AbbVie/Abbott, Eli Lilly, galapagos; E. Kristianslund, None; B. Leeb, Sandoz, AbbVie/Abbott, Eli Lilly, Roche, Grunenthal, Biogen, Celgene; G. Lukina, AbbVie/Abbott, Eli Lilly, Merck/MSD, Roche, Pfizer; D. Nordstrom, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, Novartis, Pfizer, Roche, UCB; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; M. Pombo-Suarez, Janssen, Eli Lilly, Merck/MSD, Novartis, sanofi; Z. Rotar, Pfizer, AbbVie/Abbott, Eli Lilly; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; D. Courvoisier, None; A. Finckh, Pfizer, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, AbbVie/Abbott, galapagos, mylan, UCB, Viatris.

