Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0265: In Patients with Rheumatoid Arthritis, Clinical Examination of the Feet Is Important for Understanding Individual Disease Burden, but Does Not Provoke a Change in Therapy in Most Cases
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NL
Nicolai Leuchten, MD
University Clinical Center Carl Gustav Carus
Dresden, Germany
Abstract Poster Presenter(s)
Nicolai Leuchten, Christoph Weinert and Martin Aringer, University Medical Center and Faculty of Medicine TU Dresden, Dresden, Germany
Background/Purpose: Disease activity scores like CDAI, SDAI, or DAS28, are essential for measuring rheumatoid arthritis (RA) disease activity. These scores do not contain foot joints. However, foot pain is an important aspect of morbidity for RA patients, which leads to reduced mobility and quality of life.
Methods: Joint scores of 545 RA outpatients, performed at each visit, were followed 08/2004 - 12/2020. Informed consent was obtained from every patient.
The standard CDAI cut-offs used were CDAI ≤ 2.8 for remission (REM), >2.8 - 10.0 for low disease activity (LDA), >10.0 - 22.0 for moderate disease activity (MDA) and >22.0 for high disease activity (HDA).
Active foot joint involvement (FJI) was defined as having at least one swollen (SJ) or tender (TJ) foot joint in patients who ever had palpable synovitis in any of these joints. The visits were grouped according to the number of total swollen joints (SJ 0-28). The mean Physician Global Assessment of disease activity (PhGA) was calculated for each SJ group and the difference between the mean PhGA in patients with and without FJI was calculated for each SJ group (ΔPhGA).
Results: A total of 7,577 visits of 545 patients were included, for a mean of 14 visits per patient. 413 (75.8%) of the patients were female, the mean age at visit was 59 years, mean disease duration was 13 years, 284 (52.1%) were ACPA positive. The median CDAI at visit was 4.3 (interquartile range IQR 1.6 - 9.0), the median DAS28 was 2.56 (IQR 1.89 - 3.37), the median CRP was 2.5 (IQR 1.0 - 6.2) mg/L.
At 2,853 (37.7%) visits the patient was in REM, at 3,134 (41.4%) visits in LDA, at 1,265 (16.7%) visits in MDA and at 324 (4.3%) visits in HDA.
Patients with FJI were more active and scored higher on CDAI (9.1 vs. 3.4, p< 0.001) and DAS28 (3.19 vs. 2.45, p< 0.001), even though these indices do not include the foot joints. This was mainly due to higher patient global assessment of disease activity (3.1 vs. 2.0, p< 0.001).
Involvement of the feet also led to a higher physician estimate of global disease activity. The mean difference of PhGA between patients with and without involvement of their feet (ΔPhGA) was meaningful for joint counts 0- 10, with an unweighted mean ΔPhGA of 0.273.
Of the 217 patients with FJI, 3,776 visits were analyzed. For patients with FJI, the potential change in disease activity was calculated by subtracting ΔPhGA from the CDAI at each visit. For 3,662 (97.0%) of the visits, this did not result in a change of disease activity state. For 90 (2.4%) visits, disease activity changed from LDA to REM, for 21 (0.6%) from MDA to LDA, and for only 3 (0.1%) visits from HDA to MDA.
At 83 (1.1%) visits of a total of 59 (10.8%) patients, only swollen foot joints without tender foot joints were recorded.
Conclusion: Patients with foot involvement are more active and score disproportionally high on disease activity indices, even though these do not include foot joints.
While patients suffer significantly from foot involvement, underestimating disease activity by not assessing the feet would have changed the disease activity state for 3.1% of the visits only.
Palpable synovitis without tenderness, which would be missed by performing only a metatarsophalangeal squeeze test, was found at one in a hundred visits.
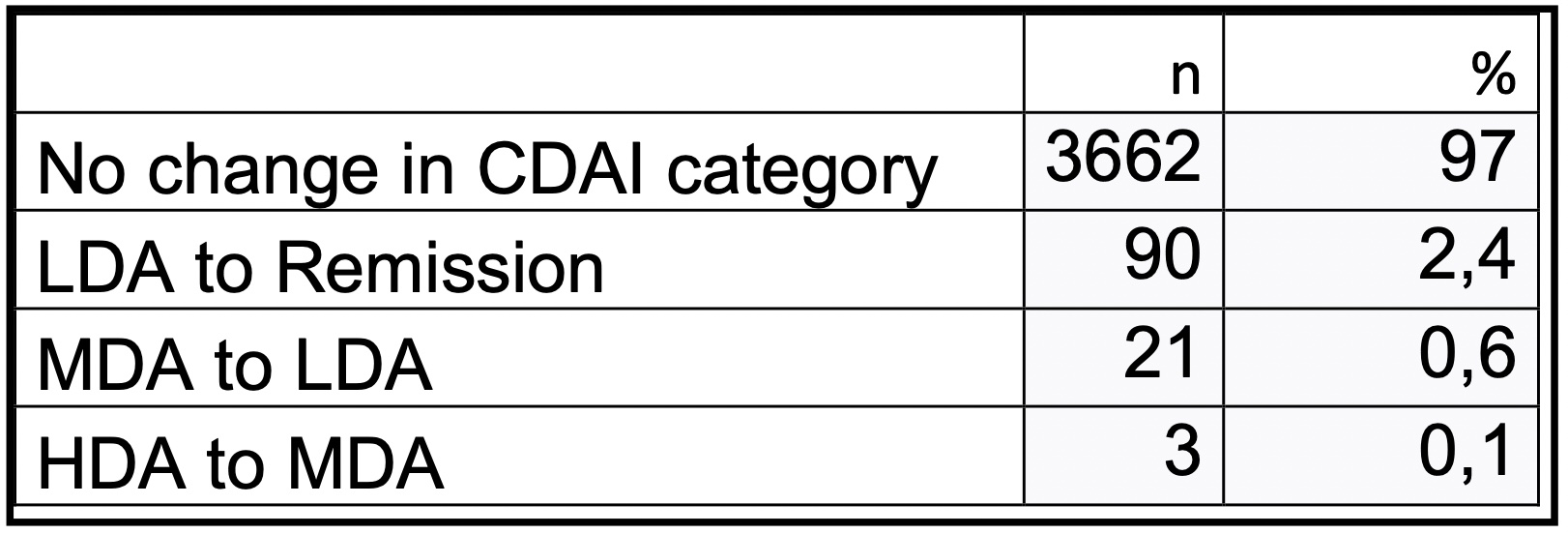 Change in CDAI disease activity state in patients with FJI when ΔPhDA is subtracted from CDAI. (LDA: low, MDA: moderate, HDA: high disease activity).
Change in CDAI disease activity state in patients with FJI when ΔPhDA is subtracted from CDAI. (LDA: low, MDA: moderate, HDA: high disease activity).
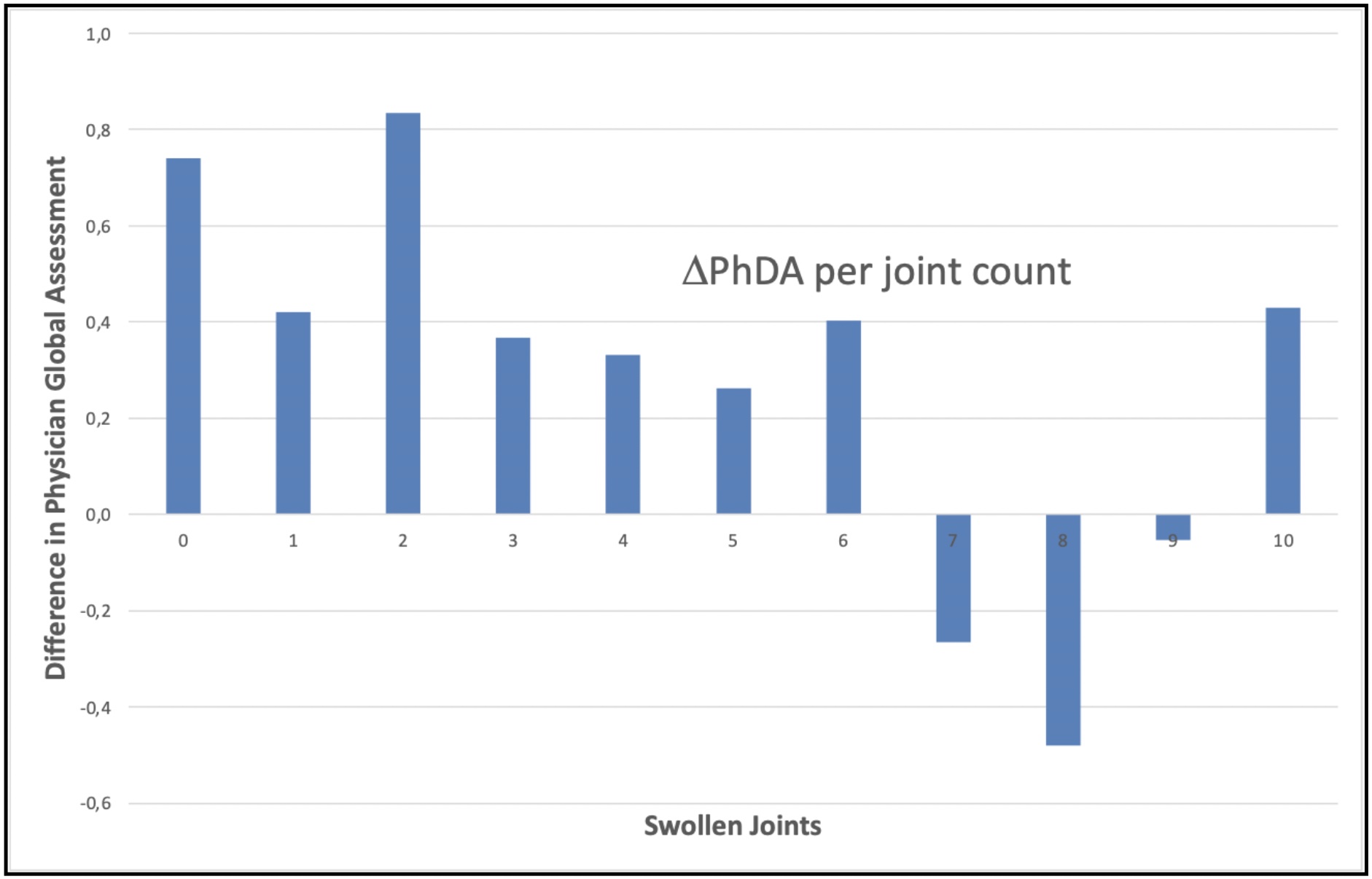 Difference of mean Physician Global Disease Activity (PhDA) in patients with/without foot involvement per joint count (ΔPhDA).
Difference of mean Physician Global Disease Activity (PhDA) in patients with/without foot involvement per joint count (ΔPhDA).
Disclosures: N. Leuchten, Roche, Novartis, GlaxoSmithKlein(GSK), UCB, AstraZeneca, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, Pfizer; C. Weinert, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka.
Background/Purpose: Disease activity scores like CDAI, SDAI, or DAS28, are essential for measuring rheumatoid arthritis (RA) disease activity. These scores do not contain foot joints. However, foot pain is an important aspect of morbidity for RA patients, which leads to reduced mobility and quality of life.
Methods: Joint scores of 545 RA outpatients, performed at each visit, were followed 08/2004 - 12/2020. Informed consent was obtained from every patient.
The standard CDAI cut-offs used were CDAI ≤ 2.8 for remission (REM), >2.8 - 10.0 for low disease activity (LDA), >10.0 - 22.0 for moderate disease activity (MDA) and >22.0 for high disease activity (HDA).
Active foot joint involvement (FJI) was defined as having at least one swollen (SJ) or tender (TJ) foot joint in patients who ever had palpable synovitis in any of these joints. The visits were grouped according to the number of total swollen joints (SJ 0-28). The mean Physician Global Assessment of disease activity (PhGA) was calculated for each SJ group and the difference between the mean PhGA in patients with and without FJI was calculated for each SJ group (ΔPhGA).
Results: A total of 7,577 visits of 545 patients were included, for a mean of 14 visits per patient. 413 (75.8%) of the patients were female, the mean age at visit was 59 years, mean disease duration was 13 years, 284 (52.1%) were ACPA positive. The median CDAI at visit was 4.3 (interquartile range IQR 1.6 - 9.0), the median DAS28 was 2.56 (IQR 1.89 - 3.37), the median CRP was 2.5 (IQR 1.0 - 6.2) mg/L.
At 2,853 (37.7%) visits the patient was in REM, at 3,134 (41.4%) visits in LDA, at 1,265 (16.7%) visits in MDA and at 324 (4.3%) visits in HDA.
Patients with FJI were more active and scored higher on CDAI (9.1 vs. 3.4, p< 0.001) and DAS28 (3.19 vs. 2.45, p< 0.001), even though these indices do not include the foot joints. This was mainly due to higher patient global assessment of disease activity (3.1 vs. 2.0, p< 0.001).
Involvement of the feet also led to a higher physician estimate of global disease activity. The mean difference of PhGA between patients with and without involvement of their feet (ΔPhGA) was meaningful for joint counts 0- 10, with an unweighted mean ΔPhGA of 0.273.
Of the 217 patients with FJI, 3,776 visits were analyzed. For patients with FJI, the potential change in disease activity was calculated by subtracting ΔPhGA from the CDAI at each visit. For 3,662 (97.0%) of the visits, this did not result in a change of disease activity state. For 90 (2.4%) visits, disease activity changed from LDA to REM, for 21 (0.6%) from MDA to LDA, and for only 3 (0.1%) visits from HDA to MDA.
At 83 (1.1%) visits of a total of 59 (10.8%) patients, only swollen foot joints without tender foot joints were recorded.
Conclusion: Patients with foot involvement are more active and score disproportionally high on disease activity indices, even though these do not include foot joints.
While patients suffer significantly from foot involvement, underestimating disease activity by not assessing the feet would have changed the disease activity state for 3.1% of the visits only.
Palpable synovitis without tenderness, which would be missed by performing only a metatarsophalangeal squeeze test, was found at one in a hundred visits.
 Change in CDAI disease activity state in patients with FJI when ΔPhDA is subtracted from CDAI. (LDA: low, MDA: moderate, HDA: high disease activity).
Change in CDAI disease activity state in patients with FJI when ΔPhDA is subtracted from CDAI. (LDA: low, MDA: moderate, HDA: high disease activity). Difference of mean Physician Global Disease Activity (PhDA) in patients with/without foot involvement per joint count (ΔPhDA).
Difference of mean Physician Global Disease Activity (PhDA) in patients with/without foot involvement per joint count (ΔPhDA).Disclosures: N. Leuchten, Roche, Novartis, GlaxoSmithKlein(GSK), UCB, AstraZeneca, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, Pfizer; C. Weinert, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka.

