Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0360: Longitudinal Variation of Proteomic Biomarkers That Correlate with Efficacy Endpoints: Results from a Phase 3 Trial of Anifrolumab in Moderate to Severe Systemic Lupus Erythematosus
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- ML
Mark Lazarus, MD, PhD
AstraZeneca
Cambridge, United Kingdom
Abstract Poster Presenter(s)
Mark Lazarus1, Paul Newcombe1, Richard A. Furie2, Philip Brohawn3, Wendy White3, Dominic Sinibaldi3, Nicola Ferrari1, Raj Tummala3, Hussein Al-Mossawi1, Edward Vital4, Eric F. Morand5, Daniel Muthas6 and Madhu Ramaswamy3, 1AstraZeneca, Cambridge, United Kingdom, 2Northwell Health, Great Neck, NY, 3AstraZeneca, Gaithersburg, MD, 4University of Leeds, Leeds, England, United Kingdom, 5Monash University, Melbourne, Australia, 6AstraZeneca, Gothenburg, Sweden
Background/Purpose: Phase 2/3 clinical trials in patients with moderate to severe SLE have demonstrated that anifrolumab, a monoclonal antibody blocking IFNAR1, produced better clinical outcomes vs standard of care.1-3 Type I IFNs (IFN-I) have diverse immunostimulatory effects, and the biomarkers and mechanistic pathways modulated by blockade of IFN-I pathway activation with anifrolumab, as well as those associated with the observed clinical improvements, have not been studied. The aim of the present study was to identify treatment-specific proteomic PD biomarkers that correlate with BILAG-based Composite Lupus Assessment (BICLA) responder status and other efficacy and immunological endpoints in TULIP-1 (NCT02446912),2 a phase 3 randomized clinical trial of anifrolumab vs standard of care in moderate to severe SLE.
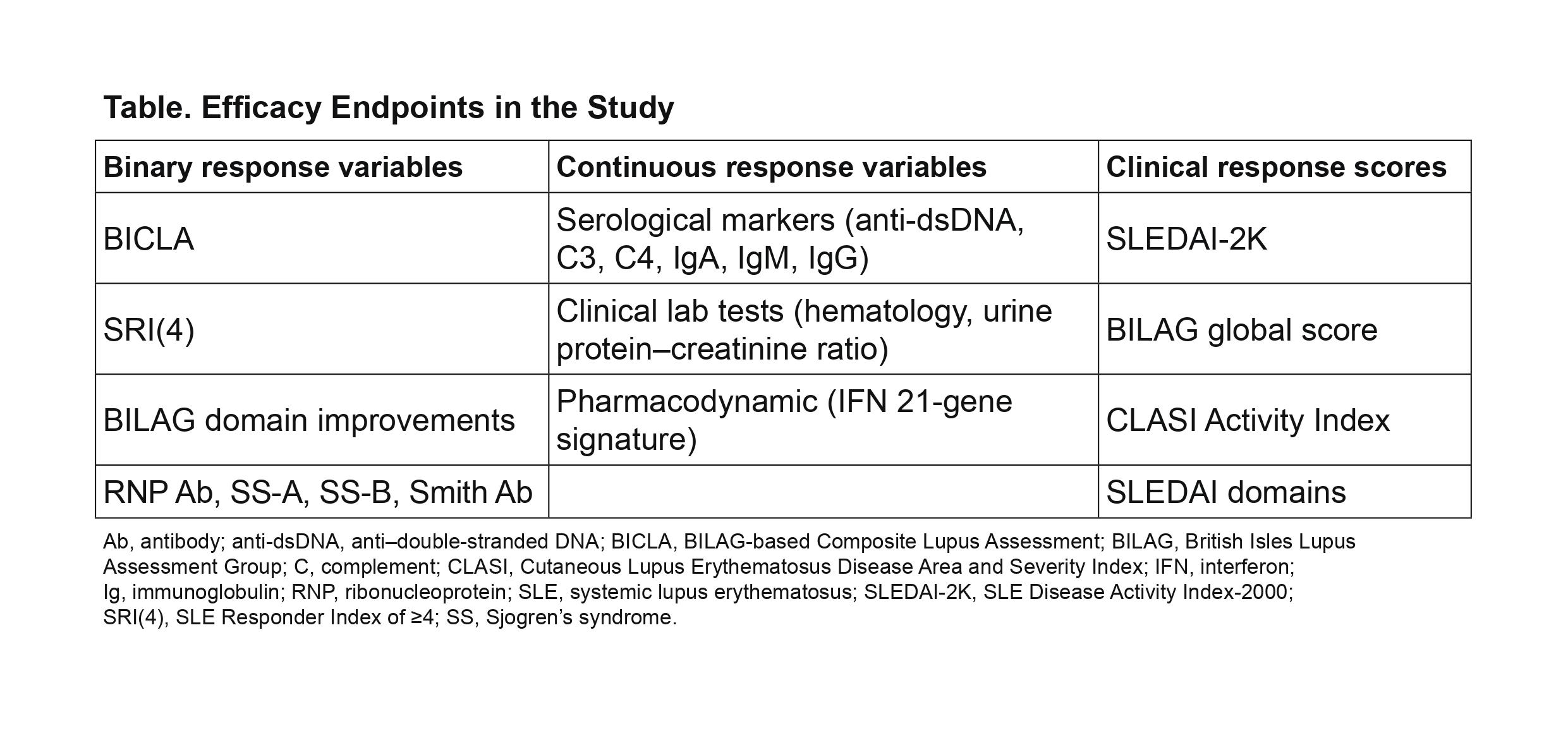
Methods: Plasma samples were assessed for 96 proteins using multiplexed Luminex assays (86 analytes) and Simoa technology (10 analytes). Longitudinal mixed-effect models determined which proteins were significantly impacted by anifrolumab vs placebo. Multiple testing was accounted for using the Benjamini-Hochberg False Discovery Rate procedure (0.05 threshold). Relationships between significantly altered serum protein concentrations and clinical changes were assessed using regression analysis and repeated measure correlation (RMCORR).4 All binary, continuous, and clinical response score measures analyzed are shown in Table.
Results: A total of 316 patients from the TULIP-1 trial, that fulfilled the 1997 ACR criteria for SLE, were included in this study. Longitudinal assessment of 1-year trajectories identified 16 proteins that were differentially associated with anifrolumab vs placebo treatment across all patients, with a larger number (29 proteins) significantly associated in the 80% of patients with high baseline IFN gene signature. Regression and RMCORR analysis of these 29 proteins revealed multiple proteins that correlated with improvements in BILAG, SLEDAI, and Cutaneous LE Disease Area and Severity Index (CLASI), key serological measures (in particular, anti–dsDNA, C3, and C4), and all hematological measures. Only MCP-2/CCL8 and TNF had changes that significantly correlated with 1-year BICLA response. Several other proteins associated with macrophage activation, including CD163, MCP-1/CCL2, MIP-3β/CCL19, and IFN-γ, were also associated with anifrolumab treatment and had changes that correlated significantly with 1-year clinical outcomes.
Conclusion: These results suggest a mechanistic role for MCP-2 and TNF in anifrolumab-associated BICLA response and that anifrolumab treatment modulates macrophage responses and activation. Anifrolumab treatment of patients with moderate to severe SLE suppresses multiple type I IFN–mediated immune proteins that correlate significantly with positive clinical outcomes.
References
1. Furie R. Arthritis Rheumatol. 2017;69:376–86.
2. Furie R. Lancet Rheumatol. 2019;1:e208–19.
3. Morand EF. N Engl J Med. 2020;382:211–21.
4. Bakdash JZ, Marusich LR. Front Psychol. 2017;8:456.
Disclosures: M. Lazarus, AstraZeneca, Novartis; P. Newcombe, AstraZeneca; R. Furie, AstraZeneca, Biogen; P. Brohawn, AstraZeneca; W. White, AstraZeneca; D. Sinibaldi, AstraZeneca, Neuraly; N. Ferrari, AstraZeneca; R. Tummala, AstraZeneca; H. Al-Mossawi, AstraZeneca; E. Vital, AstraZeneca; E. Morand, AbbVie, Amgen, AstraZeneca, Biogen, BristolMyersSquibb, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, Novartis, Servier, UCB, EMD Soreno; D. Muthas, AstraZeneca; M. Ramaswamy, AstraZeneca.
Background/Purpose: Phase 2/3 clinical trials in patients with moderate to severe SLE have demonstrated that anifrolumab, a monoclonal antibody blocking IFNAR1, produced better clinical outcomes vs standard of care.1-3 Type I IFNs (IFN-I) have diverse immunostimulatory effects, and the biomarkers and mechanistic pathways modulated by blockade of IFN-I pathway activation with anifrolumab, as well as those associated with the observed clinical improvements, have not been studied. The aim of the present study was to identify treatment-specific proteomic PD biomarkers that correlate with BILAG-based Composite Lupus Assessment (BICLA) responder status and other efficacy and immunological endpoints in TULIP-1 (NCT02446912),2 a phase 3 randomized clinical trial of anifrolumab vs standard of care in moderate to severe SLE.
Methods: Plasma samples were assessed for 96 proteins using multiplexed Luminex assays (86 analytes) and Simoa technology (10 analytes). Longitudinal mixed-effect models determined which proteins were significantly impacted by anifrolumab vs placebo. Multiple testing was accounted for using the Benjamini-Hochberg False Discovery Rate procedure (0.05 threshold). Relationships between significantly altered serum protein concentrations and clinical changes were assessed using regression analysis and repeated measure correlation (RMCORR).4 All binary, continuous, and clinical response score measures analyzed are shown in Table.
Results: A total of 316 patients from the TULIP-1 trial, that fulfilled the 1997 ACR criteria for SLE, were included in this study. Longitudinal assessment of 1-year trajectories identified 16 proteins that were differentially associated with anifrolumab vs placebo treatment across all patients, with a larger number (29 proteins) significantly associated in the 80% of patients with high baseline IFN gene signature. Regression and RMCORR analysis of these 29 proteins revealed multiple proteins that correlated with improvements in BILAG, SLEDAI, and Cutaneous LE Disease Area and Severity Index (CLASI), key serological measures (in particular, anti–dsDNA, C3, and C4), and all hematological measures. Only MCP-2/CCL8 and TNF had changes that significantly correlated with 1-year BICLA response. Several other proteins associated with macrophage activation, including CD163, MCP-1/CCL2, MIP-3β/CCL19, and IFN-γ, were also associated with anifrolumab treatment and had changes that correlated significantly with 1-year clinical outcomes.
Conclusion: These results suggest a mechanistic role for MCP-2 and TNF in anifrolumab-associated BICLA response and that anifrolumab treatment modulates macrophage responses and activation. Anifrolumab treatment of patients with moderate to severe SLE suppresses multiple type I IFN–mediated immune proteins that correlate significantly with positive clinical outcomes.
References
1. Furie R. Arthritis Rheumatol. 2017;69:376–86.
2. Furie R. Lancet Rheumatol. 2019;1:e208–19.
3. Morand EF. N Engl J Med. 2020;382:211–21.
4. Bakdash JZ, Marusich LR. Front Psychol. 2017;8:456.

Disclosures: M. Lazarus, AstraZeneca, Novartis; P. Newcombe, AstraZeneca; R. Furie, AstraZeneca, Biogen; P. Brohawn, AstraZeneca; W. White, AstraZeneca; D. Sinibaldi, AstraZeneca, Neuraly; N. Ferrari, AstraZeneca; R. Tummala, AstraZeneca; H. Al-Mossawi, AstraZeneca; E. Vital, AstraZeneca; E. Morand, AbbVie, Amgen, AstraZeneca, Biogen, BristolMyersSquibb, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, Novartis, Servier, UCB, EMD Soreno; D. Muthas, AstraZeneca; M. Ramaswamy, AstraZeneca.

