Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0261: Machine Learning and Computational Pathology Can Reveal Synovial Pathotypes in Mice and Humans
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RB
Richard Bell, PhD
Hospital for Special Surgery
New York, NY, United States
Abstract Poster Presenter(s)
Richard Bell1, Matthew Brendel2, Justin Xiang3, AMP RA/SLE Consortium1, Edward DiCarlo1, Jennifer Anolik4, Laura Donlin1, Dana Orange5, Edward Schwarz4, Lionel Ivashkiv1 and Fei Wang2, 1Hospital for Special Surgery, New York, NY, 2Weil Cornell Medical College, New York, NY, 3Horace Grant High School, New York, NY, 4University of Rochester Medical Center, Rochester, NY, 5The Rockefeller University, New York, NY
Background/Purpose: Synovial pathotyping is a critical step in understanding the etiology of inflammatory arthritis as different pathotypes have differential response to therapy. Determining these pathotypes relies on immunohistochemistry of lymphoid, myeloid and stromal cells with expert grading of histologic sections, which can be time consuming and costly. Here, we developed an automated segmentation and cell type classification pipeline to efficiently phenotype a murine inflammatory arthritis model; and demonstrate the feasibility of this analysis in human synovial biopsies.
Methods: The synovial tissue, cartilage, bone, and fatty tissue from H&E sections of healthy (n=6), mild (n=8) and severely diseased (n=5) TNF transgenic mice that develop arthritis (Training and Testing Slide) was segmented using a knee tissue segmentation model previously developed in our lab1. We then annotated bone cells (n=312), vessel cells (n=378), adipo-stromal cells (n=506), fibroblasts (n=749), chondrocytes (n=625), lymphocytes (n=467), and all other synovial lining cells (n=1675). Nuclei were segmented and cellular image features were extracted in ImageJ/Python. A stratified 5-fold cross validation strategy was used to train a Gradient Boosted Decision Tree to classify cell types and F1 scores were calculated. Another set of 68 TNF-Tg slides2 (External Validation) describing the model's sexual dimorphism were subjected to the tissue segmentation, nuclear segmentation, and cell feature extraction and cell types from the synovial tissue were inferred to validate the ability to phenotype the synovium. Also, five human synovial biopsies H&E sections from the Accelerating Medicine Partnership - Rheumatoid Arthritis consortium were annotated for 7 cell types: endothelial cell (n=117), fibroblasts (n=61), lymphocytes (n=71), neutrophils (n=93), plasma cells (n=77), stromal cells (n=57) and synovial lining cells (n=26). The same image analysis and machine learning pipeline was performed.
Results: UMAP demonstrated good separation of TNF-Tg cells and our classification model performed well (Fig 1A, B). We inferred the cell types of the remaining cells on the training and testing slides and demonstrated the tissue specificity of adipo-stromal cells and chondrocytes as the major cell type within their respective tissue (Fig1 C). When stratified by disease severity, fibroblasts, lining cells and lymphocytes followed the expected distribution (Fig 1D). In the external validation set, we also observed the expected increase in synovial lymphocytes and stromal cells in TNF-Tg female mice compared to males at earlier timepoints, but interestingly not in the fibroblast compartment, which suggested a differential etiology (Fig 2A-C). Our pipeline is also able to confidently classify 7 cell types in human RA synovial biopsies, suggesting that we might be able to incorporate our process into other synovial pathotyping pipelines (Fig 3).
Conclusion: We developed a computational pathology cell classification pipeline that confidently identifies cell types in murine and human inflammatory arthritis H&E stained tissue sections. This will allow for efficient pathotyping of diseased tissues to elucidate varying etiologies.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290230-1-ANY.jpg width=440 height=294.60223675881 border=0 style=border-style: none;>
.jpg) Figure 2. Lymphocytes and Synovial Lining Cells but not fibroblasts are sexually dimorphic in the TNF-Tg model. Cell type prediction in a sexually dimorphic model of inflammatory arthritis describes the previously demonstrated difference in inflammatory lymphoid infiltrates (here as Lymphocytes, A) and synovial hyperplasia (B, Synovial Lining cells). Interestingly, there is no difference in fibroblast cells counts between TNF-Tg male or female mice at any timepoint. Two-Way ANOVA, ***p < 0.001, **p < 0.01
Figure 2. Lymphocytes and Synovial Lining Cells but not fibroblasts are sexually dimorphic in the TNF-Tg model. Cell type prediction in a sexually dimorphic model of inflammatory arthritis describes the previously demonstrated difference in inflammatory lymphoid infiltrates (here as Lymphocytes, A) and synovial hyperplasia (B, Synovial Lining cells). Interestingly, there is no difference in fibroblast cells counts between TNF-Tg male or female mice at any timepoint. Two-Way ANOVA, ***p < 0.001, **p < 0.01
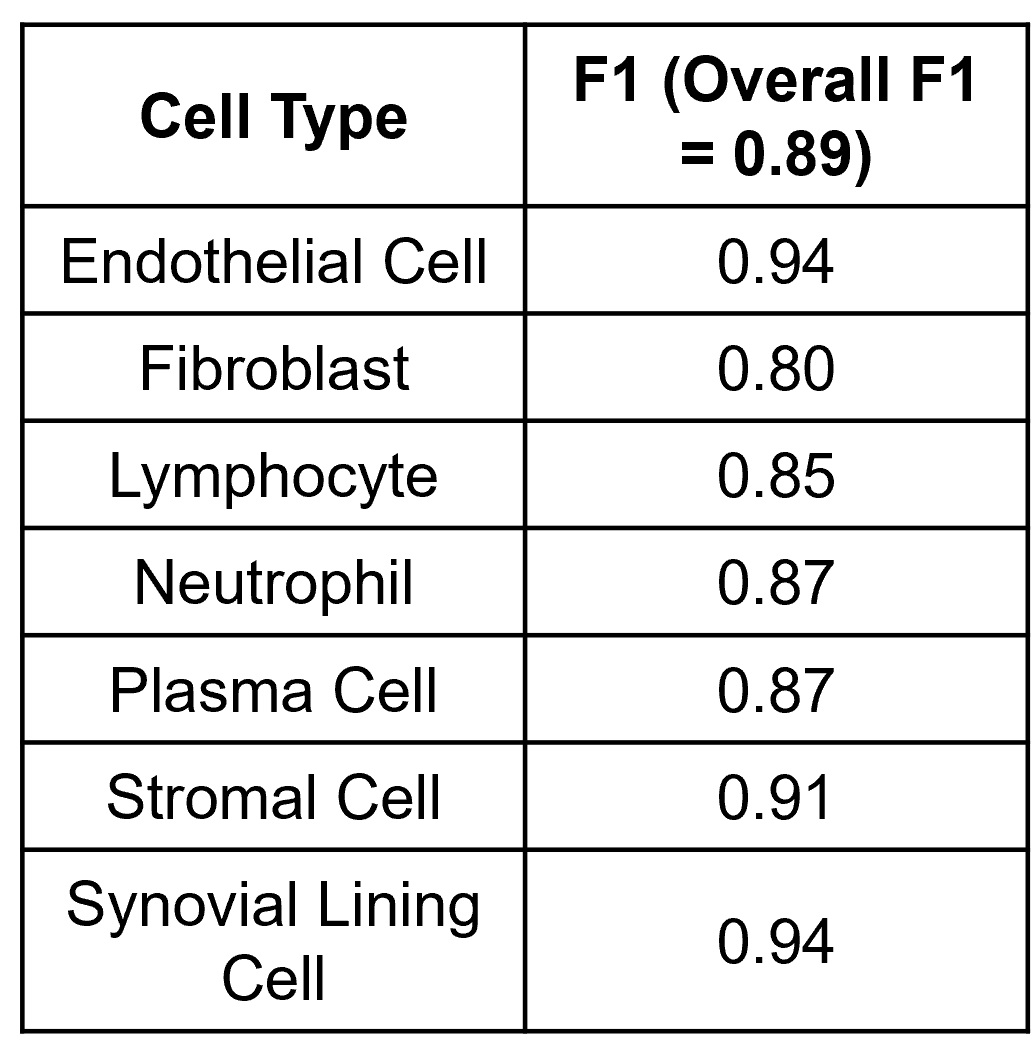 Figure 3. A gradient boosted decision tree classifies cells from human synovial tissue biopsies with high performance. Utilizing our pipeline developed on murine tissue, we classify seven important cell types for synovial pathotyping with good overall and cell specific performance.
Figure 3. A gradient boosted decision tree classifies cells from human synovial tissue biopsies with high performance. Utilizing our pipeline developed on murine tissue, we classify seven important cell types for synovial pathotyping with good overall and cell specific performance.
Disclosures: R. Bell, None; M. Brendel, None; J. Xiang, None; A. Consortium, None; E. DiCarlo, None; J. Anolik, None; L. Donlin, None; D. Orange, None; E. Schwarz, None; L. Ivashkiv, None; F. Wang, None.
Background/Purpose: Synovial pathotyping is a critical step in understanding the etiology of inflammatory arthritis as different pathotypes have differential response to therapy. Determining these pathotypes relies on immunohistochemistry of lymphoid, myeloid and stromal cells with expert grading of histologic sections, which can be time consuming and costly. Here, we developed an automated segmentation and cell type classification pipeline to efficiently phenotype a murine inflammatory arthritis model; and demonstrate the feasibility of this analysis in human synovial biopsies.
Methods: The synovial tissue, cartilage, bone, and fatty tissue from H&E sections of healthy (n=6), mild (n=8) and severely diseased (n=5) TNF transgenic mice that develop arthritis (Training and Testing Slide) was segmented using a knee tissue segmentation model previously developed in our lab1. We then annotated bone cells (n=312), vessel cells (n=378), adipo-stromal cells (n=506), fibroblasts (n=749), chondrocytes (n=625), lymphocytes (n=467), and all other synovial lining cells (n=1675). Nuclei were segmented and cellular image features were extracted in ImageJ/Python. A stratified 5-fold cross validation strategy was used to train a Gradient Boosted Decision Tree to classify cell types and F1 scores were calculated. Another set of 68 TNF-Tg slides2 (External Validation) describing the model's sexual dimorphism were subjected to the tissue segmentation, nuclear segmentation, and cell feature extraction and cell types from the synovial tissue were inferred to validate the ability to phenotype the synovium. Also, five human synovial biopsies H&E sections from the Accelerating Medicine Partnership - Rheumatoid Arthritis consortium were annotated for 7 cell types: endothelial cell (n=117), fibroblasts (n=61), lymphocytes (n=71), neutrophils (n=93), plasma cells (n=77), stromal cells (n=57) and synovial lining cells (n=26). The same image analysis and machine learning pipeline was performed.
Results: UMAP demonstrated good separation of TNF-Tg cells and our classification model performed well (Fig 1A, B). We inferred the cell types of the remaining cells on the training and testing slides and demonstrated the tissue specificity of adipo-stromal cells and chondrocytes as the major cell type within their respective tissue (Fig1 C). When stratified by disease severity, fibroblasts, lining cells and lymphocytes followed the expected distribution (Fig 1D). In the external validation set, we also observed the expected increase in synovial lymphocytes and stromal cells in TNF-Tg female mice compared to males at earlier timepoints, but interestingly not in the fibroblast compartment, which suggested a differential etiology (Fig 2A-C). Our pipeline is also able to confidently classify 7 cell types in human RA synovial biopsies, suggesting that we might be able to incorporate our process into other synovial pathotyping pipelines (Fig 3).
Conclusion: We developed a computational pathology cell classification pipeline that confidently identifies cell types in murine and human inflammatory arthritis H&E stained tissue sections. This will allow for efficient pathotyping of diseased tissues to elucidate varying etiologies.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290230-1-ANY.jpg width=440 height=294.60223675881 border=0 style=border-style: none;>
Figure 1. Cell type classification model successfully identifies important cell types in inflammatory arthritis. Utilizing (UMAP) to project in 2D, we see good separation of Lymphocytes, Bone Cells, Chondrocytes, and Adipo-stromal Cells (A). The gradient boosted decision tree performs well with an overall F1 of 0.83 ± 0.12 (M ± SD) and the class specific F1 is shown in (B). We then predicted the cell class (>75% predicted probability) of all other cells identified on the slides (~300,000). To provide quality control, we looked at the predicted cells within the Fat tissue and Cartilage/Meniscus and our model appropriately classifies the majority cells within these tissue as fat cells or chondrocytes, respectively (C). We next looked at the cells within the synovium and found that our model classifies fibroblast, lymphocytes and other synovial lining cells well, and their distribution meets the expectation in healthy mild and severely disease knees (D).
.jpg) Figure 2. Lymphocytes and Synovial Lining Cells but not fibroblasts are sexually dimorphic in the TNF-Tg model. Cell type prediction in a sexually dimorphic model of inflammatory arthritis describes the previously demonstrated difference in inflammatory lymphoid infiltrates (here as Lymphocytes, A) and synovial hyperplasia (B, Synovial Lining cells). Interestingly, there is no difference in fibroblast cells counts between TNF-Tg male or female mice at any timepoint. Two-Way ANOVA, ***p < 0.001, **p < 0.01
Figure 2. Lymphocytes and Synovial Lining Cells but not fibroblasts are sexually dimorphic in the TNF-Tg model. Cell type prediction in a sexually dimorphic model of inflammatory arthritis describes the previously demonstrated difference in inflammatory lymphoid infiltrates (here as Lymphocytes, A) and synovial hyperplasia (B, Synovial Lining cells). Interestingly, there is no difference in fibroblast cells counts between TNF-Tg male or female mice at any timepoint. Two-Way ANOVA, ***p < 0.001, **p < 0.01 Figure 3. A gradient boosted decision tree classifies cells from human synovial tissue biopsies with high performance. Utilizing our pipeline developed on murine tissue, we classify seven important cell types for synovial pathotyping with good overall and cell specific performance.
Figure 3. A gradient boosted decision tree classifies cells from human synovial tissue biopsies with high performance. Utilizing our pipeline developed on murine tissue, we classify seven important cell types for synovial pathotyping with good overall and cell specific performance.Disclosures: R. Bell, None; M. Brendel, None; J. Xiang, None; A. Consortium, None; E. DiCarlo, None; J. Anolik, None; L. Donlin, None; D. Orange, None; E. Schwarz, None; L. Ivashkiv, None; F. Wang, None.

