Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0292: Malignancy in the Upadacitinib Clinical Trial Programs for Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Andrea Rubbert-Roth, MD
Cantonal Hospital St Gallen
St Gallen, Switzerland
Abstract Poster Presenter(s)
Andrea Rubbert-Roth1, Adriana Kakehasi2, Tsutomu Takeuchi3, Marc Schmalzing4, Hannah Palac5, Jianzhong Liu5, Samuel Anyanwu5, Ralph Lippe6 and Jeffrey Curtis7, 1Division of Rheumatology, Cantonal Clinic St Gallen, St.Gallen, Switzerland, 2Federal University of Minas Gerais, Hospital das Clínicas, Belo Horizonte, Brazil, 3Keio University and Saitama Medical University, Tokyo, Japan, 4University Hospital Wuerzburg, Wuerzburg, Germany, 5AbbVie, Inc., North Chicago, IL, 6AbbVie, Inc, Wiesbaden, Germany, 7University of Alabama at Birmingham, Birmingham, AL
Background/Purpose: Increased risk of malignancies has been associated with chronic inflammation, as well as immunosuppressive and immunomodulatory therapies. The objective of this analysis is to describe events of malignancy in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS) from the SELECT clinical trial programs for upadacitinib (UPA), a Janus kinase inhibitor, versus active comparators (adalimumab 40 mg [ADA] and methotrexate [MTX]).
Methods: Safety data (cut-off: 30 June 2021) from 9 phase 3 UPA trials were compiled for RA (6 trials), PsA (2 trials), and AS (1 trial; phase 2b/3). Treatment-emergent adverse events (TEAEs; onset on or after first dose and ≤30 days after last dose for UPA 15 mg [approved dose for rheumatological indications] and MTX or ≤70 days after last dose for ADA) of malignancy were summarized for RA (pooled UPA15, ADA [SELECT-COMPARE study only], and MTX [SELECT-EARLY study only]), PsA (pooled UPA15 and ADA [SELECT-PsA 1 study only]), and AS (UPA15). TEAEs are reported as exposure-adjusted event rates (EAERs; events/100 patient years [E/100 PY]). Age-gender adjusted standard incidence ratios (SIR) were calculated for RA, due to a sufficient number of reports, using the SEER database for the general population. Univariable Cox proportional hazards regression models were used to assess the impact of known risk factors on rates of malignancy excluding non-melanoma skin cancer (NMSC) in RA and PsA in the UPA15 treatment groups.
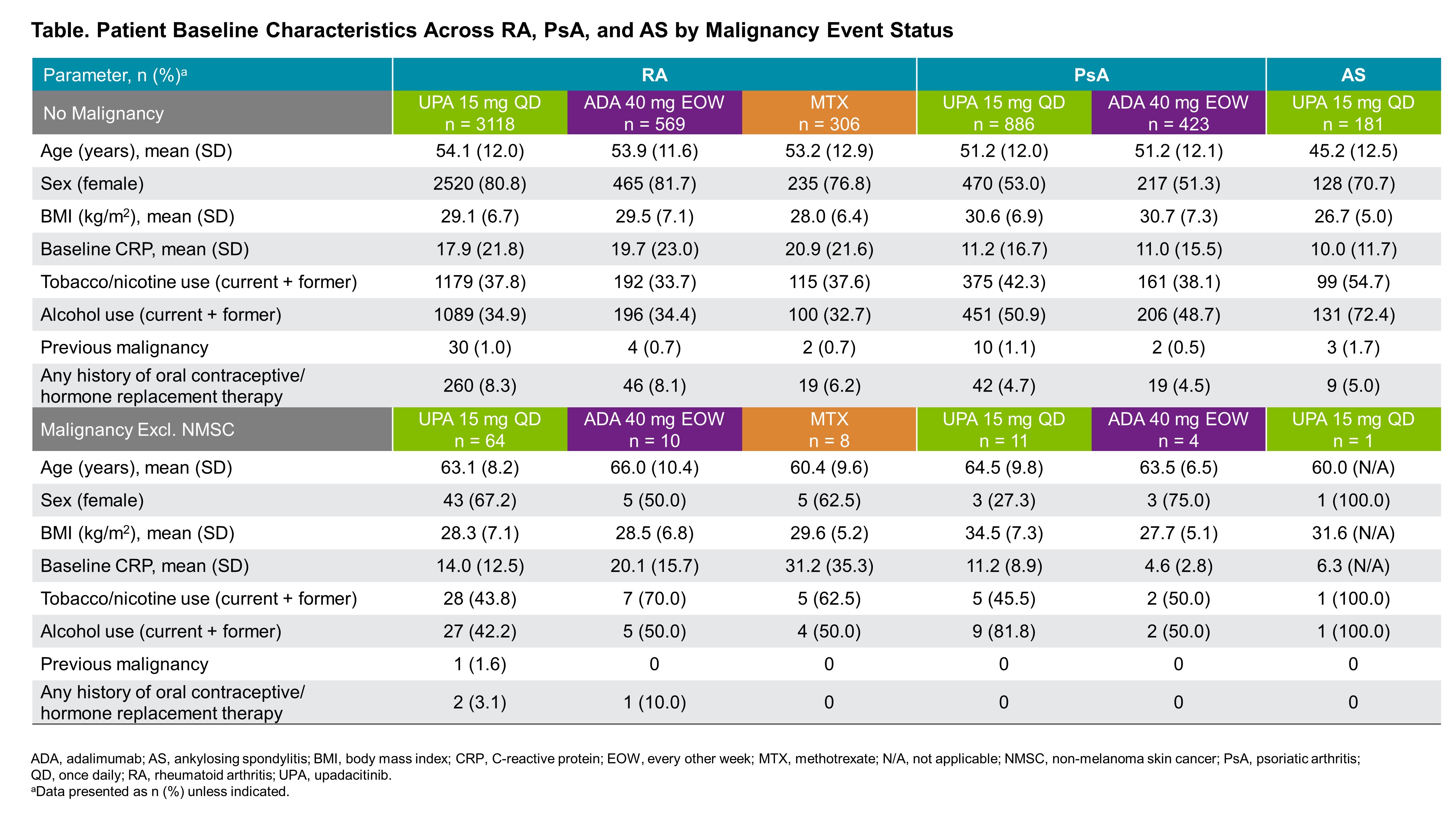
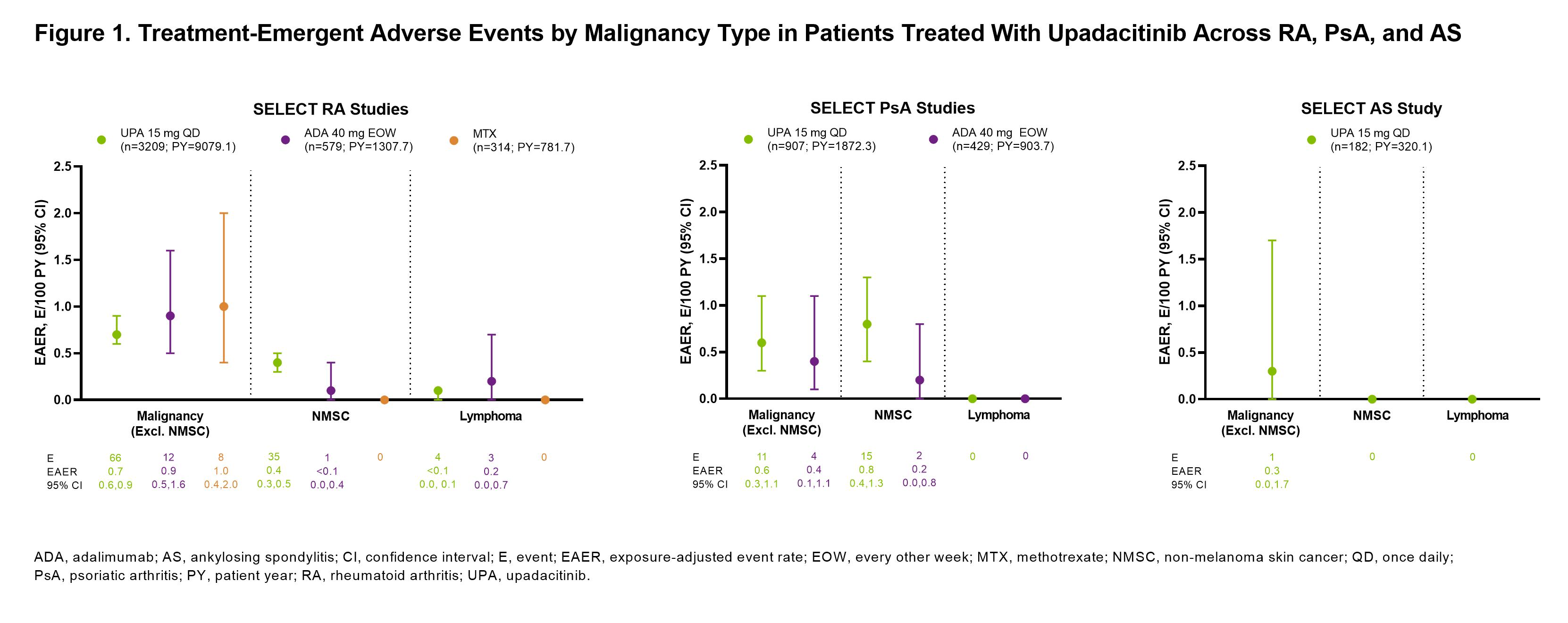
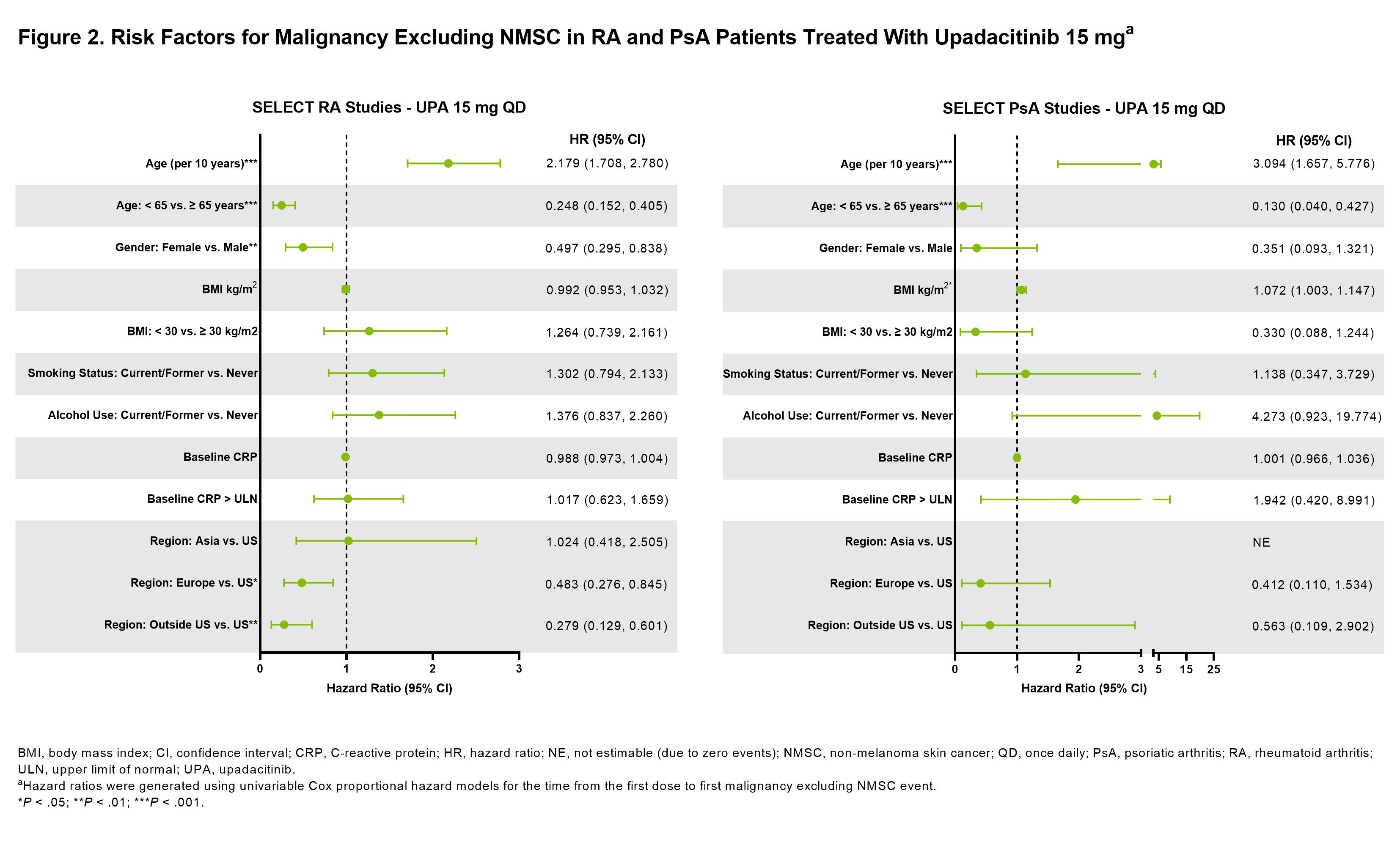
Results: Across RA, PsA, and AS, a total of 4298 patients received ≥1 dose of UPA15 (RA, n=3209; PsA, n=907; AS, n=182), totaling 11,271.5 PY of exposure (Figure 1; baseline characteristics provided in Table 1). In RA, event rates of malignancy excluding NMSC were similar between UPA15, ADA, and MTX; rates of NMSC were higher with UPA15 compared to ADA and MTX (Figure 1). The most common types of malignancy excluding NMSC were breast cancer and lung cancer. The age-gender adjusted SIR (95% CI) for malignancy excluding NMSC in RA was 1.0 (0.80-1.24) for UPA15. In PsA, rates of NMSC and malignancy excluding NMSC were similar between UPA15 and ADA. In AS, a single event of malignancy excluding NMSC was reported with UPA15. Lymphoma was infrequent in RA, with no events in PsA or AS. In the univariable regression models for UPA15, being older, male, and in the US compared to other regions were associated with increased risk of malignancy excluding NMSC in patients with RA, while being older and having higher BMI were associated with increased risk in patients with PsA (Figure 2).
Conclusion: Event rates of malignancy excluding NMSC were generally similar between UPA15, ADA, and MTX and consistent across RA, PsA, and AS. NMSC was more frequent in patients receiving UPA15 than ADA or MTX. Due to low rates of malignancy, findings from the univariable regression models will need to be confirmed in future studies.
Disclosures: A. Rubbert-Roth, None; A. Kakehasi, AbbVie, Amgen, Janssen, UCB, Pfizer, Eli Lilly, Novartis, Sandoz, Fresenius Kabi, Organon; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; M. Schmalzing, Novartis, AbbVie, AstraZeneca, Chugai/Roche, Janssen, Gilead, Boehringer/Ingelheim, Celgene, Medac, UCB, Sandoz; H. Palac, AbbVie; J. Liu, AbbVie; S. Anyanwu, AbbVie; R. Lippe, AbbVie; J. Curtis, AbbVie, Inc., Amgen, Bristol Myers Squibb, CorEvitas, Janssen, Labcorp, Eli Lilly, Novartis, Pfizer, Sanofi/Regeneron, UCB.
Background/Purpose: Increased risk of malignancies has been associated with chronic inflammation, as well as immunosuppressive and immunomodulatory therapies. The objective of this analysis is to describe events of malignancy in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS) from the SELECT clinical trial programs for upadacitinib (UPA), a Janus kinase inhibitor, versus active comparators (adalimumab 40 mg [ADA] and methotrexate [MTX]).
Methods: Safety data (cut-off: 30 June 2021) from 9 phase 3 UPA trials were compiled for RA (6 trials), PsA (2 trials), and AS (1 trial; phase 2b/3). Treatment-emergent adverse events (TEAEs; onset on or after first dose and ≤30 days after last dose for UPA 15 mg [approved dose for rheumatological indications] and MTX or ≤70 days after last dose for ADA) of malignancy were summarized for RA (pooled UPA15, ADA [SELECT-COMPARE study only], and MTX [SELECT-EARLY study only]), PsA (pooled UPA15 and ADA [SELECT-PsA 1 study only]), and AS (UPA15). TEAEs are reported as exposure-adjusted event rates (EAERs; events/100 patient years [E/100 PY]). Age-gender adjusted standard incidence ratios (SIR) were calculated for RA, due to a sufficient number of reports, using the SEER database for the general population. Univariable Cox proportional hazards regression models were used to assess the impact of known risk factors on rates of malignancy excluding non-melanoma skin cancer (NMSC) in RA and PsA in the UPA15 treatment groups.
Results: Across RA, PsA, and AS, a total of 4298 patients received ≥1 dose of UPA15 (RA, n=3209; PsA, n=907; AS, n=182), totaling 11,271.5 PY of exposure (Figure 1; baseline characteristics provided in Table 1). In RA, event rates of malignancy excluding NMSC were similar between UPA15, ADA, and MTX; rates of NMSC were higher with UPA15 compared to ADA and MTX (Figure 1). The most common types of malignancy excluding NMSC were breast cancer and lung cancer. The age-gender adjusted SIR (95% CI) for malignancy excluding NMSC in RA was 1.0 (0.80-1.24) for UPA15. In PsA, rates of NMSC and malignancy excluding NMSC were similar between UPA15 and ADA. In AS, a single event of malignancy excluding NMSC was reported with UPA15. Lymphoma was infrequent in RA, with no events in PsA or AS. In the univariable regression models for UPA15, being older, male, and in the US compared to other regions were associated with increased risk of malignancy excluding NMSC in patients with RA, while being older and having higher BMI were associated with increased risk in patients with PsA (Figure 2).
Conclusion: Event rates of malignancy excluding NMSC were generally similar between UPA15, ADA, and MTX and consistent across RA, PsA, and AS. NMSC was more frequent in patients receiving UPA15 than ADA or MTX. Due to low rates of malignancy, findings from the univariable regression models will need to be confirmed in future studies.



Disclosures: A. Rubbert-Roth, None; A. Kakehasi, AbbVie, Amgen, Janssen, UCB, Pfizer, Eli Lilly, Novartis, Sandoz, Fresenius Kabi, Organon; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; M. Schmalzing, Novartis, AbbVie, AstraZeneca, Chugai/Roche, Janssen, Gilead, Boehringer/Ingelheim, Celgene, Medac, UCB, Sandoz; H. Palac, AbbVie; J. Liu, AbbVie; S. Anyanwu, AbbVie; R. Lippe, AbbVie; J. Curtis, AbbVie, Inc., Amgen, Bristol Myers Squibb, CorEvitas, Janssen, Labcorp, Eli Lilly, Novartis, Pfizer, Sanofi/Regeneron, UCB.

