Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0335: Multiplexed Mass Cytometry of Cutaneous Lupus Erythematosus and Dermatomyositis Skin: An In-depth B Cell Directed Immunoprofile
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MO
Mariko Ogawa-Momohara, MD, PhD
Nagoya University Graduate School of Medicine
Nagoya, Japan
Abstract Poster Presenter(s)
Mariko Ogawa-Momohara1, Thomas Vazquez2, Meena Sharma2, Josh Dan3, Grant Sprow3 and Victoria Werth3, 1Nagoya University Graduate School of Medicine, Nagoya, Japan, 2Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 3Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia
Background/Purpose: Cutaneous lupus erythematosus (CLE) and dermatomyositis (DM) are both characterized histologically by interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate, requiring clinical correlation to distinguish the two entities. Although some studies suggest that B cell targeting therapies are effective for the cutaneous manifestations of CLE and DM, responses to anti-CD20 treatment differ between CLE and DM and are independent from the systemic response. We therefore sought to characterize the B/plasma cell compartment in CLE and DM skin to better understand the cutaneous immunopathogenesis of these autoimmune connective tissues diseases.
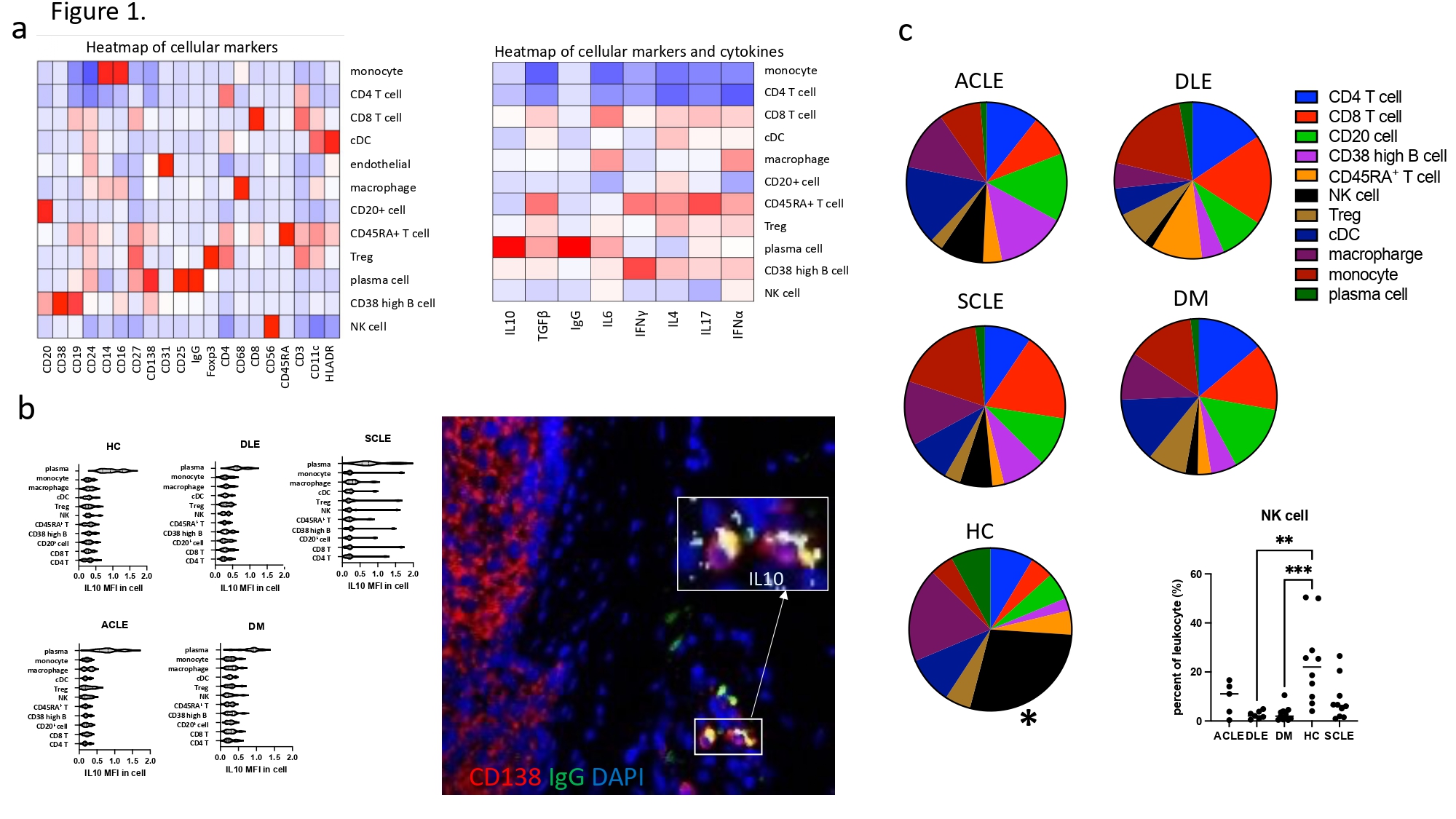
Methods: We recruited patients from our prospective CLE and DM databases at the University of Pennsylvania with Institutional Review Board approval. Age-matched healthy controls (HC) were selected from the Penn Skin Biology and Disease Resource Center. We performed imaging mass cytometry on 43 archived, lesional skin biopsies (11 DM, 5 ACLE, 7 DLE, 10 SCLE and 10 HC) using 28 distinct cell markers, cytokines, and immunoglobulins to identify the immunophenotype in each group (figure 1).
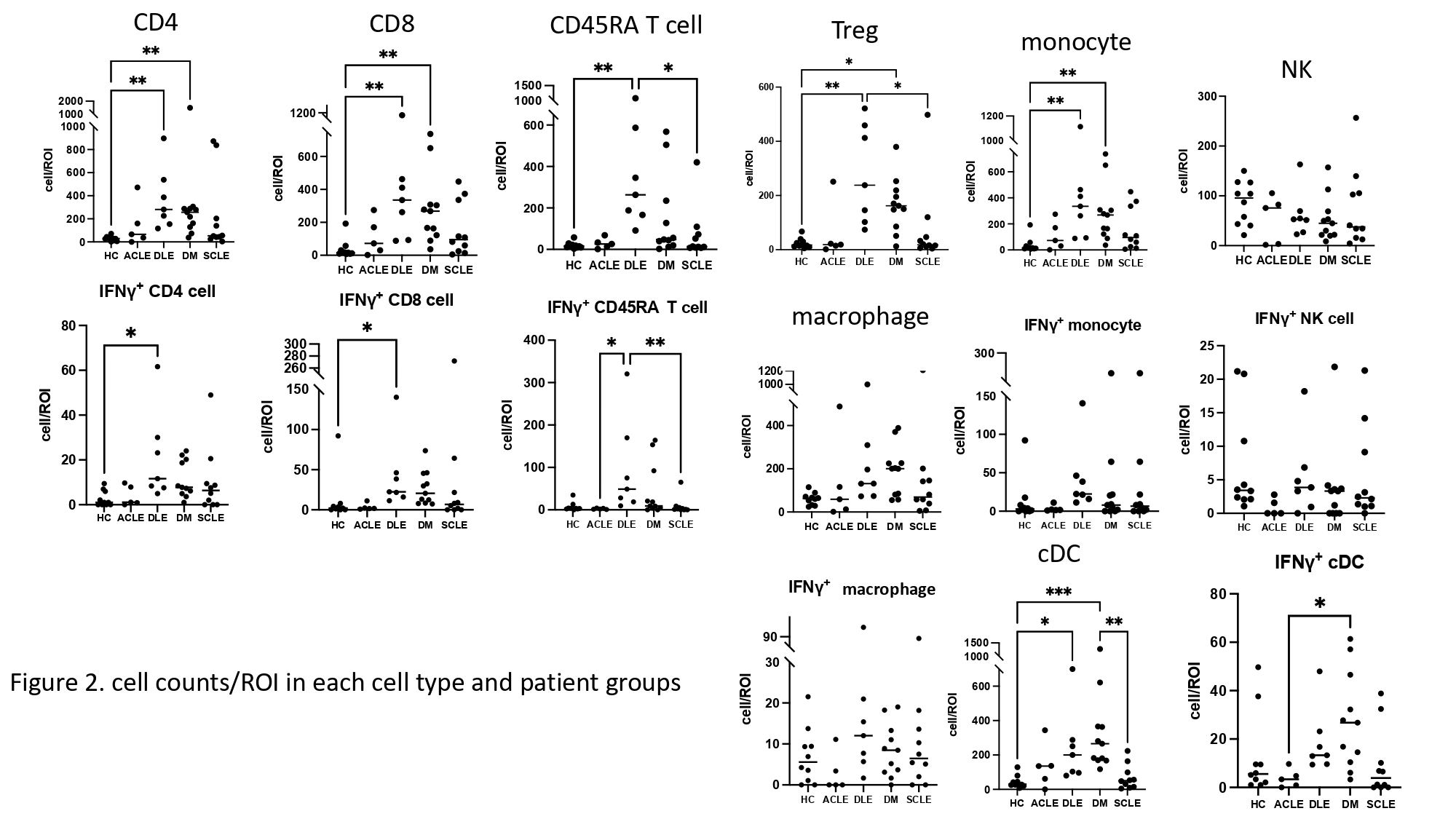
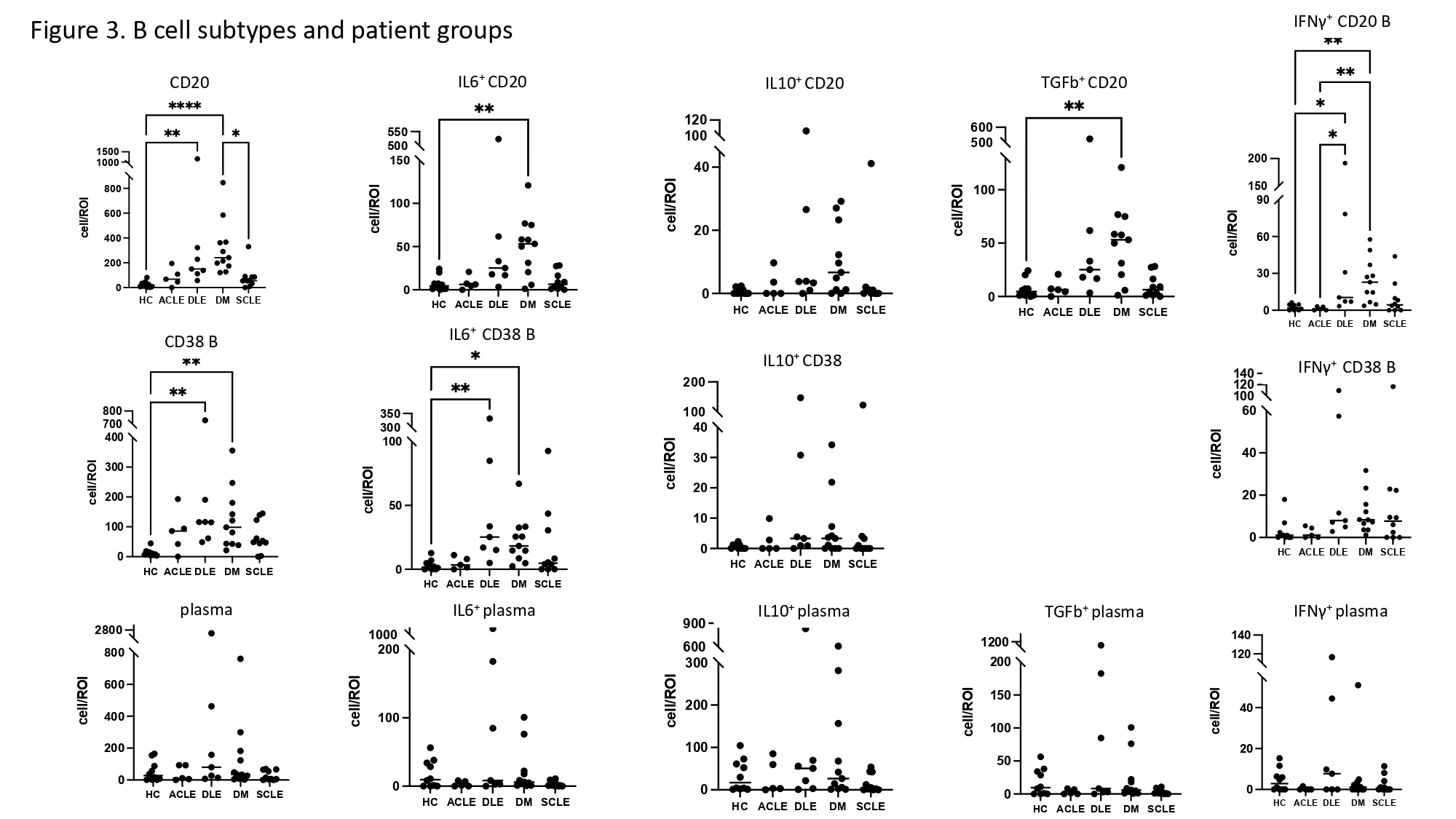
Results: We found 11 significant lymphocyte cell groups among all samples that included 3 B cell groups (CD138+ CD27+ IgGhigh plasma cell, CD38high+ B cell, and other CD20+ cells), 4 T cell groups (CD4+ T cell, CD8+ T cell, Treg, and CD45RAhighT cell), monocytes, classical dendritic cells (cDC), NK cells, and macrophages (figure 1). Cell counts of T cells/region of interest (ROI) (CD4+, CD8+, Treg and CD45RAhigh) were most upregulated in DLE compared with HC (p< 0.01). CD20+ cells were increased in DM compared to HC (p< 0.001). CD38high B cell counts/ROI were significantly increased in DM and DLE compared to HC (p< 0.01). cDCs were increased in DM compared to HC (p< 0.001) (figure 2, 3). The percentage of NK cells among all lymphocytes cells was highest in HC (figure 1). IL10 was highly expressed in plasma cells relative to other cell types. The number of plasma cells in DM and ACLE was positively correlated with total skin IL10 intensity (r=0.83, r=0.97) and IgG intensity (r=0.9, r=0.92) but not in DLE and SCLE. IFNg+ CD4 T cell, IFNγ+ CD8 T cells, IL6+ CD38high B cells were increased in DLE relative to HC (p< .05, p< .05, p< .01) (figure 2, 3). Between CLE subgroups, CD45RA T, Treg, and IFNγ+ CD45RA T cell/ROI were significantly higher in DLE than SCLE (p< 0.05, p< 0.05, p< 0.01). IFNγ+ CD45RA T cell and IFNγ+ CD20 B cell are higher in DLE than ACLE (p< 0.05).
Conclusion: We identified several differences in the B and T lymphocyte compartments in CLE and DM. DLE skin demonstrated increased T cells and CD38high B cell, whereas CD20+ B cell and cDCs were increased in DM. The primary IL10 producing cell in DM CLE and HC was the plasma cell in the skin.
Disclosures: M. Ogawa-Momohara, None; T. Vazquez, None; M. Sharma, None; J. Dan, None; G. Sprow, None; V. Werth, GlaxoSmithKline, CLASI.
Background/Purpose: Cutaneous lupus erythematosus (CLE) and dermatomyositis (DM) are both characterized histologically by interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate, requiring clinical correlation to distinguish the two entities. Although some studies suggest that B cell targeting therapies are effective for the cutaneous manifestations of CLE and DM, responses to anti-CD20 treatment differ between CLE and DM and are independent from the systemic response. We therefore sought to characterize the B/plasma cell compartment in CLE and DM skin to better understand the cutaneous immunopathogenesis of these autoimmune connective tissues diseases.
Methods: We recruited patients from our prospective CLE and DM databases at the University of Pennsylvania with Institutional Review Board approval. Age-matched healthy controls (HC) were selected from the Penn Skin Biology and Disease Resource Center. We performed imaging mass cytometry on 43 archived, lesional skin biopsies (11 DM, 5 ACLE, 7 DLE, 10 SCLE and 10 HC) using 28 distinct cell markers, cytokines, and immunoglobulins to identify the immunophenotype in each group (figure 1).
Results: We found 11 significant lymphocyte cell groups among all samples that included 3 B cell groups (CD138+ CD27+ IgGhigh plasma cell, CD38high+ B cell, and other CD20+ cells), 4 T cell groups (CD4+ T cell, CD8+ T cell, Treg, and CD45RAhighT cell), monocytes, classical dendritic cells (cDC), NK cells, and macrophages (figure 1). Cell counts of T cells/region of interest (ROI) (CD4+, CD8+, Treg and CD45RAhigh) were most upregulated in DLE compared with HC (p< 0.01). CD20+ cells were increased in DM compared to HC (p< 0.001). CD38high B cell counts/ROI were significantly increased in DM and DLE compared to HC (p< 0.01). cDCs were increased in DM compared to HC (p< 0.001) (figure 2, 3). The percentage of NK cells among all lymphocytes cells was highest in HC (figure 1). IL10 was highly expressed in plasma cells relative to other cell types. The number of plasma cells in DM and ACLE was positively correlated with total skin IL10 intensity (r=0.83, r=0.97) and IgG intensity (r=0.9, r=0.92) but not in DLE and SCLE. IFNg+ CD4 T cell, IFNγ+ CD8 T cells, IL6+ CD38high B cells were increased in DLE relative to HC (p< .05, p< .05, p< .01) (figure 2, 3). Between CLE subgroups, CD45RA T, Treg, and IFNγ+ CD45RA T cell/ROI were significantly higher in DLE than SCLE (p< 0.05, p< 0.05, p< 0.01). IFNγ+ CD45RA T cell and IFNγ+ CD20 B cell are higher in DLE than ACLE (p< 0.05).
Conclusion: We identified several differences in the B and T lymphocyte compartments in CLE and DM. DLE skin demonstrated increased T cells and CD38high B cell, whereas CD20+ B cell and cDCs were increased in DM. The primary IL10 producing cell in DM CLE and HC was the plasma cell in the skin.



Disclosures: M. Ogawa-Momohara, None; T. Vazquez, None; M. Sharma, None; J. Dan, None; G. Sprow, None; V. Werth, GlaxoSmithKline, CLASI.

