Back
Poster Session A
Vasculitis
Session: (0432–0457) Vasculitis – ANCA-Associated Poster I: Epidemiology, Outcomes, and Classification
0441: Patient-Reported Outcomes in ANCA-Associated Vasculitis: Interactions Between Patients' and Physicians' Perspectives in a Mexican Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JH
José Joel Hurtado Arias, MD
Universidad Nacional Autónoma de México/ Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
Ciudad de México, Federal District, Mexico
Abstract Poster Presenter(s)
José Joel Hurtado-Arias1, Isabela Ramírez-Mulhern2, Javier Merayo-Chalico3, Carlos A. Gonzalez-Martinez4, Ana Barrera-Vargas2 and Andrea Hinojosa-Azaola1, 1Department of Immunology and Rheumatology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Ciudad de México, Mexico, 2Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Ciudad de México, Mexico, 3Instituto Nacional de Ciencias Medicas y Nutricion "Salvador Zubiran", Ciudad de México, Mexico, 4Department of Urology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Ciudad de México, Mexico
Background/Purpose: The antineutrophil cytoplasm antibody (ANCA) associated vasculitis (AAV) are a group of complex chronic diseases resulting in morbidity, accumulated organ damage, treatment burden, and risk of relapses. A key aspect of the outcome measures is to incorporate the patients' views to determine the impact of the disease. This study aimed to evaluate associations between the domains of the ANCA-associated vasculitis Patient-Reported Outcome (AAV-PRO) instrument and clinical variables.
Methods: Patients with Granulomatosis with Polyangiitis (GPA), Microscopic Polyangiitis (MPA), Eosinophilic Granulomatosis with Polyangiitis (EGPA), or renal-limited vasculitis (RLV) according to definitions of the Chapel Hill Consensus Nomenclature and/or the ACR 1990 Classification Criteria were recruited in the Rheumatology consult of a tertiary care center in Mexico City. Demographic, clinical, serologic, and treatment-related variables were retrieved. Disease activity and damage were evaluated with the Birmingham Vasculitis Activity Score for GPA (BVAS/WG) and the Vasculitis Damage Index (VDI), respectively. Patient and Physician Global Assessments (PtGA and PhGA, respectively) in a 0-100 scale were also evaluated. All patients completed the AAV-PRO questionnaire, and sexual function in male patients was assessed with the International Index of Erectile Function (IIEF-5) questionnaire. Descriptive statistics were performed, correlations between variables were calculated using Pearson and Spearman coefficients, whereas differences between groups were analyzed with the Mann-Whitney U test.
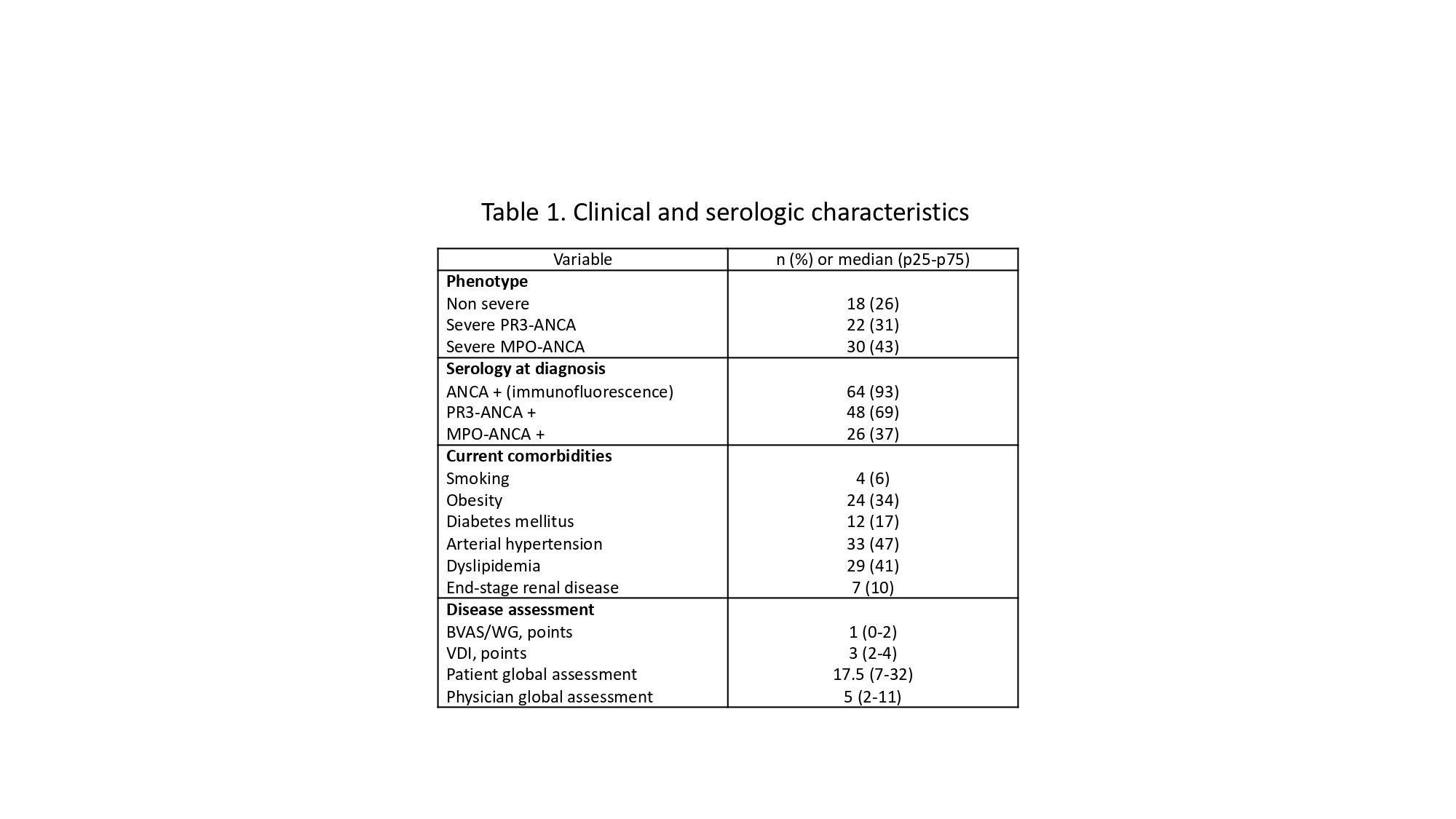
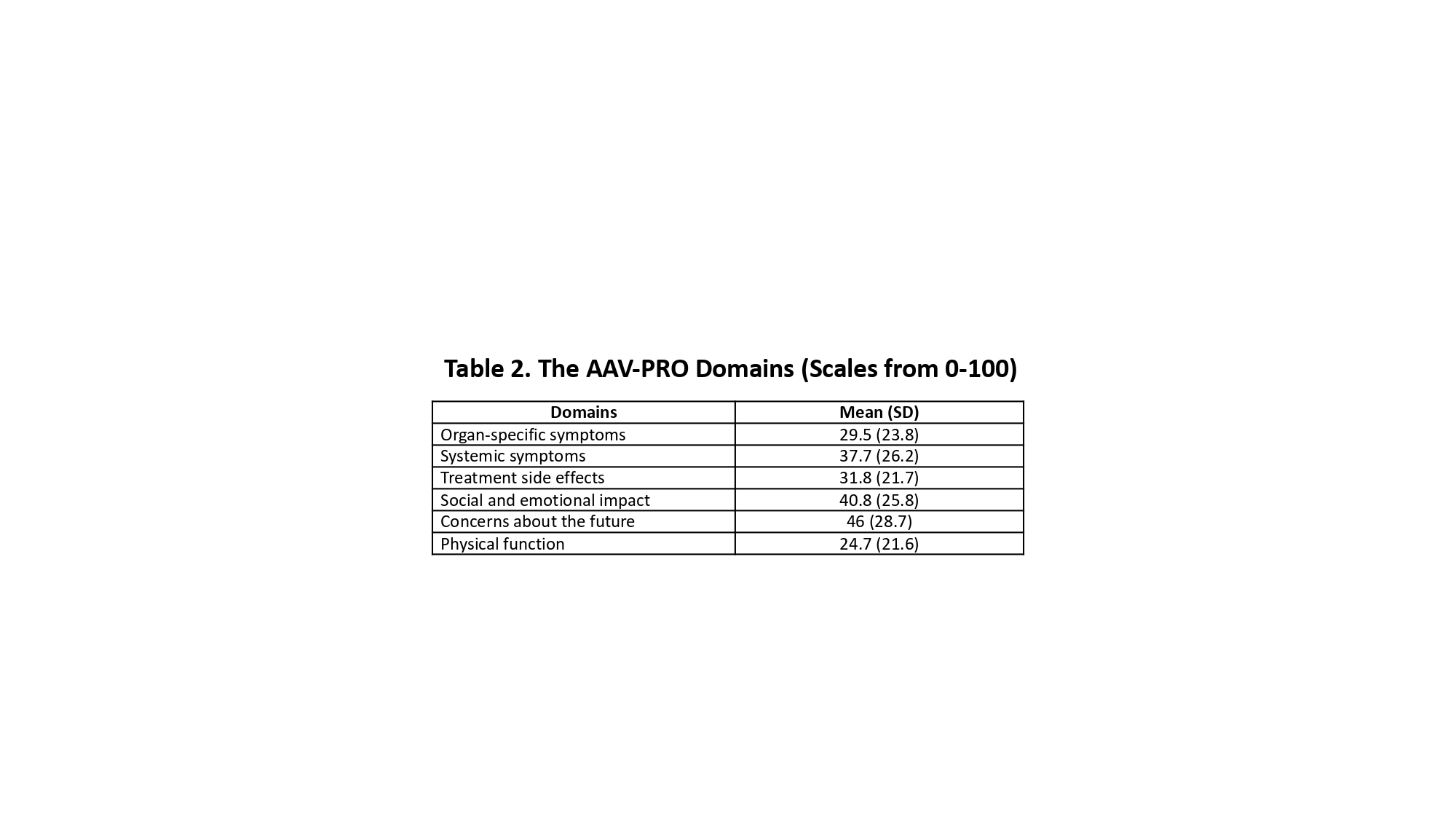
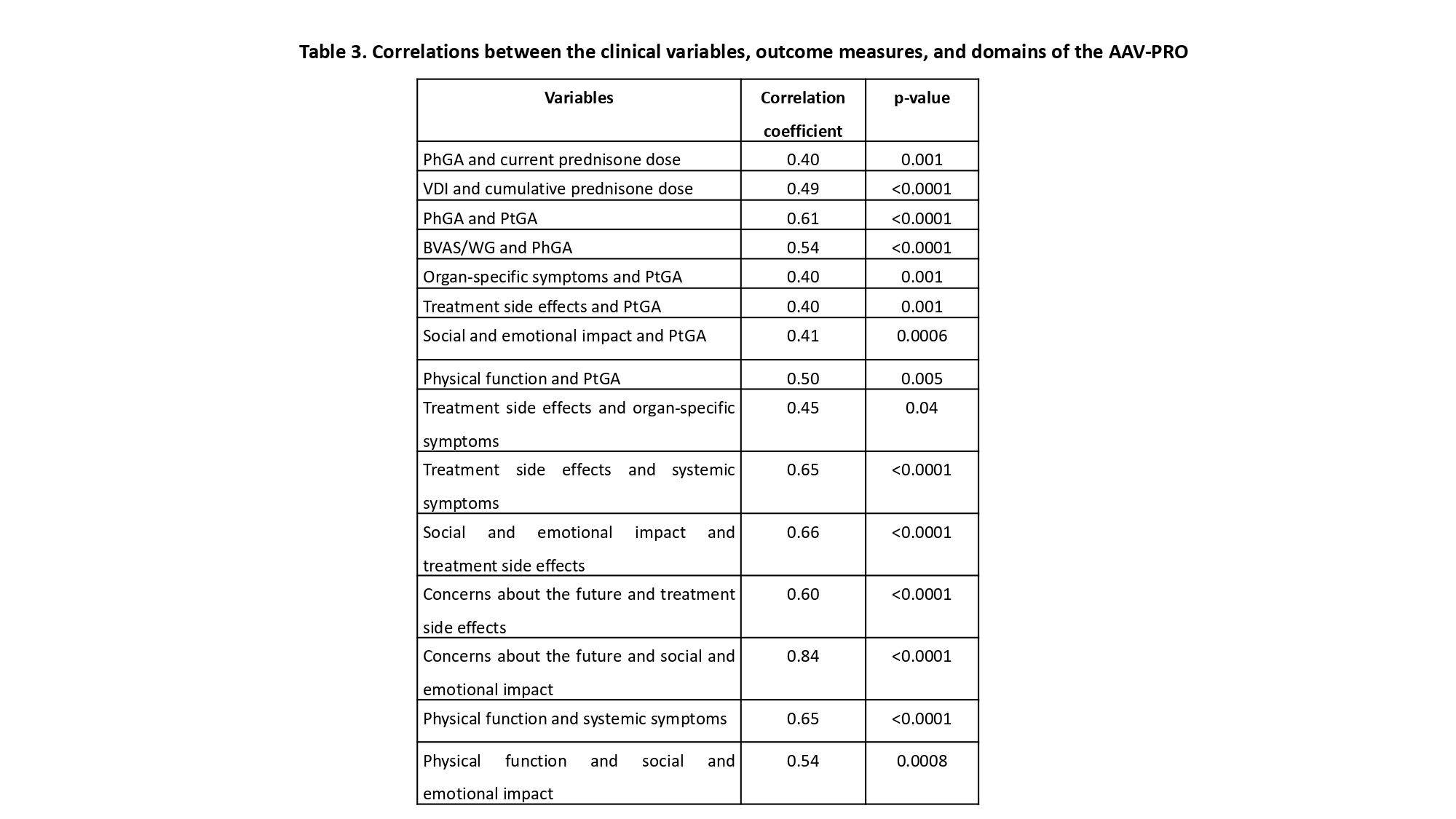
Results: Seventy patients (44 women and 26 men) were included, with a median age of 53.5 years (IQR 43-61), disease duration of 82 months (34-135), 58 (83%) had GPA, 5 (7%) EGPA, 5 (7%) RLV, and 2 (3%) MPA diagnosis. Table 1 summarizes the clinical and serologic characteristics, whereas Table 2 depicts the AAV-PRO domains (scales from 0-100 with higher scores representing higher intensity of the symptoms). 17/24 (70.8%) of men who completed the IIEF-5 questionnaire had some degree of erectile dysfunction. Correlations between the clinical variables, outcome measures, and domains of the AAV-PRO are displayed in Table 3. Significant differences were found in the following domains (median (IQR)) of the AAV-PRO according to gender: treatment side effects (20 (10-35) in men vs 35 (17.5-50) in women, p=0.04); age: treatment side effects (40 (25-55) in < 50 years-old vs 20 (10-40) in > 50 years-old, p=0.005), and concerns about the future (55 (45-85) vs 35 (10-70), respectively, p=0.02), and finally, according to disease duration: treatment side effects (40 (25-50) in patients with < 5 years vs 20 (10-45) in those with > 5 years, p=0.03). No correlations were observed between the IIEF-5 score and other variables.
Conclusion: In this cohort of AAV patients with low disease activity, correlations were found between the specific domains of the AAV-PRO and other outcome measures. Differences were found between some of the AAV-PRO domains according to gender, age, and disease duration. It is essential to consider person-centered outcomes in the clinical care of AAV patients.
Disclosures: J. Hurtado-Arias, None; I. Ramírez-Mulhern, None; J. Merayo-Chalico, Janssen, Sanofi; C. Gonzalez-Martinez, None; A. Barrera-Vargas, AstraZeneca, Sanofi; A. Hinojosa-Azaola, None.
Background/Purpose: The antineutrophil cytoplasm antibody (ANCA) associated vasculitis (AAV) are a group of complex chronic diseases resulting in morbidity, accumulated organ damage, treatment burden, and risk of relapses. A key aspect of the outcome measures is to incorporate the patients' views to determine the impact of the disease. This study aimed to evaluate associations between the domains of the ANCA-associated vasculitis Patient-Reported Outcome (AAV-PRO) instrument and clinical variables.
Methods: Patients with Granulomatosis with Polyangiitis (GPA), Microscopic Polyangiitis (MPA), Eosinophilic Granulomatosis with Polyangiitis (EGPA), or renal-limited vasculitis (RLV) according to definitions of the Chapel Hill Consensus Nomenclature and/or the ACR 1990 Classification Criteria were recruited in the Rheumatology consult of a tertiary care center in Mexico City. Demographic, clinical, serologic, and treatment-related variables were retrieved. Disease activity and damage were evaluated with the Birmingham Vasculitis Activity Score for GPA (BVAS/WG) and the Vasculitis Damage Index (VDI), respectively. Patient and Physician Global Assessments (PtGA and PhGA, respectively) in a 0-100 scale were also evaluated. All patients completed the AAV-PRO questionnaire, and sexual function in male patients was assessed with the International Index of Erectile Function (IIEF-5) questionnaire. Descriptive statistics were performed, correlations between variables were calculated using Pearson and Spearman coefficients, whereas differences between groups were analyzed with the Mann-Whitney U test.
Results: Seventy patients (44 women and 26 men) were included, with a median age of 53.5 years (IQR 43-61), disease duration of 82 months (34-135), 58 (83%) had GPA, 5 (7%) EGPA, 5 (7%) RLV, and 2 (3%) MPA diagnosis. Table 1 summarizes the clinical and serologic characteristics, whereas Table 2 depicts the AAV-PRO domains (scales from 0-100 with higher scores representing higher intensity of the symptoms). 17/24 (70.8%) of men who completed the IIEF-5 questionnaire had some degree of erectile dysfunction. Correlations between the clinical variables, outcome measures, and domains of the AAV-PRO are displayed in Table 3. Significant differences were found in the following domains (median (IQR)) of the AAV-PRO according to gender: treatment side effects (20 (10-35) in men vs 35 (17.5-50) in women, p=0.04); age: treatment side effects (40 (25-55) in < 50 years-old vs 20 (10-40) in > 50 years-old, p=0.005), and concerns about the future (55 (45-85) vs 35 (10-70), respectively, p=0.02), and finally, according to disease duration: treatment side effects (40 (25-50) in patients with < 5 years vs 20 (10-45) in those with > 5 years, p=0.03). No correlations were observed between the IIEF-5 score and other variables.
Conclusion: In this cohort of AAV patients with low disease activity, correlations were found between the specific domains of the AAV-PRO and other outcome measures. Differences were found between some of the AAV-PRO domains according to gender, age, and disease duration. It is essential to consider person-centered outcomes in the clinical care of AAV patients.



Disclosures: J. Hurtado-Arias, None; I. Ramírez-Mulhern, None; J. Merayo-Chalico, Janssen, Sanofi; C. Gonzalez-Martinez, None; A. Barrera-Vargas, AstraZeneca, Sanofi; A. Hinojosa-Azaola, None.

