Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0427: Recapture Rates with Ixekizumab After Withdrawal of Therapy in Patients with Axial Spondyloarthritis: Results at Week 104 from a Randomized Placebo-controlled Withdrawal Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Lianne S. Gensler, MD
Professor of Medicine
University of California San Francisco
San Francisco, CA, United States
Abstract Poster Presenter(s)
Robert Landewé1, Denis Poddubnyy2, Proton Rahman3, Rebecca Bolce4, Soyi Liu-Leage5, Jeffrey Lisse6, So Young Park7 and Lianne Gensler8, 1Amsterdam University Medical Center, Meerssen, Netherlands, 2Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 3Memorial University, St. John's, NL, Canada, 4Eli Lilly and Company, Cincinnati, OH, 5Eli Lilly, Neuilly sur Seine, France, 6Eli Lilly and Company, Tucson, AZ, 7Eli Lilly and Company, Indianapolis, IN, 8Department of Medicine, Division of Rheumatology, University of California San Francisco, San Francisco, CA
Background/Purpose: Here, we describe the final results of the first study of patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis re-randomized to either placebo (ixekizumab withdrawal) or ixekizumab, who experienced a flare and recaptured response either before or after open-label retreatment during COAST-Y.
Methods: COAST-Y (NCT03129100) is a phase 3 extension study that included a double-blind, placebo-controlled, randomized withdrawal-retreatment period (RWP) through 104 weeks. Patients who achieved remission (Ankylosing Spondylitis Disease Activity Score (ASDAS)< 1.3 (inactive disease; ID) at least once at week 16 or 20, and < 2.1 (low disease activity; LDA) at both visits) were randomized 2:1 at week 24 to continue ixekizumab or withdraw to placebo. Patients who subsequently flared were switched to open-label ixekizumab Q2W or Q4W at the next visit. The proportion of patients who recaptured ASDAS LDA and ID were summarized for patients who experienced flare.
Results: A total of 155 patients entered the RWP (placebo, N=53; ixekizumab Q4W, N=48; ixekizumab Q2W, N=54) and 138 completed week 104. Thirty-six percent of patients re-randomized to placebo never experienced a flare through 104 weeks. Twenty-eight placebo-treated patients experienced a flare during weeks 24-104; of those, 4/28 (14%) recaptured LDA before retreatment with open-label ixekizumab, while 23/28 (82%) recaptured LDA and 19/28 (68%) met ID after switching to open-label ixekizumab retreatment.
Conclusion: The majority of patients withdrawn from ixekizumab to placebo recaptured at least LDA and over half met ID with ixekizumab retreatment. This may provide insight for ixekizumab therapy for patients who require a short interruption in treatment.
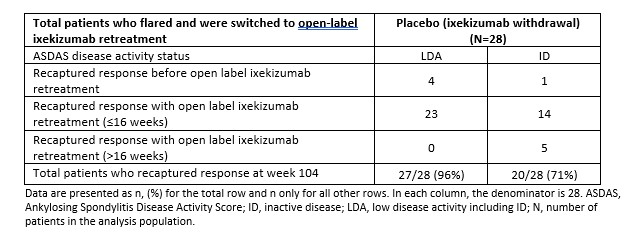 Table 1. Recapture of first treatment response before or after switching to open label IXE through 104 weeks among placebo (ixekizumab withdrawal)-treated patients who experienced a flare and retreated.
Table 1. Recapture of first treatment response before or after switching to open label IXE through 104 weeks among placebo (ixekizumab withdrawal)-treated patients who experienced a flare and retreated.
Disclosures: R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; R. Bolce, Eli Lilly; S. Liu-Leage, Eli Lilly and Company; J. Lisse, Eli Lilly and Company; S. Park, Eli Lilly and Company; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake.
Background/Purpose: Here, we describe the final results of the first study of patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis re-randomized to either placebo (ixekizumab withdrawal) or ixekizumab, who experienced a flare and recaptured response either before or after open-label retreatment during COAST-Y.
Methods: COAST-Y (NCT03129100) is a phase 3 extension study that included a double-blind, placebo-controlled, randomized withdrawal-retreatment period (RWP) through 104 weeks. Patients who achieved remission (Ankylosing Spondylitis Disease Activity Score (ASDAS)< 1.3 (inactive disease; ID) at least once at week 16 or 20, and < 2.1 (low disease activity; LDA) at both visits) were randomized 2:1 at week 24 to continue ixekizumab or withdraw to placebo. Patients who subsequently flared were switched to open-label ixekizumab Q2W or Q4W at the next visit. The proportion of patients who recaptured ASDAS LDA and ID were summarized for patients who experienced flare.
Results: A total of 155 patients entered the RWP (placebo, N=53; ixekizumab Q4W, N=48; ixekizumab Q2W, N=54) and 138 completed week 104. Thirty-six percent of patients re-randomized to placebo never experienced a flare through 104 weeks. Twenty-eight placebo-treated patients experienced a flare during weeks 24-104; of those, 4/28 (14%) recaptured LDA before retreatment with open-label ixekizumab, while 23/28 (82%) recaptured LDA and 19/28 (68%) met ID after switching to open-label ixekizumab retreatment.
Conclusion: The majority of patients withdrawn from ixekizumab to placebo recaptured at least LDA and over half met ID with ixekizumab retreatment. This may provide insight for ixekizumab therapy for patients who require a short interruption in treatment.
 Table 1. Recapture of first treatment response before or after switching to open label IXE through 104 weeks among placebo (ixekizumab withdrawal)-treated patients who experienced a flare and retreated.
Table 1. Recapture of first treatment response before or after switching to open label IXE through 104 weeks among placebo (ixekizumab withdrawal)-treated patients who experienced a flare and retreated.Disclosures: R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; R. Bolce, Eli Lilly; S. Liu-Leage, Eli Lilly and Company; J. Lisse, Eli Lilly and Company; S. Park, Eli Lilly and Company; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake.

