Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0294: Safety and Efficacy of Upadacitinib in Patients with Rheumatoid Arthritis and Inadequate Response or Intolerance to Biologic DMARDs: Results Through 5 Years from the SELECT-BEYOND Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Roy Fleischmann, MD
Metroplex Clinical Research Center and University of Texas Southwestern Medical Center
Dallas, TX, United States
Abstract Poster Presenter(s)
Roy Fleischmann1, Sebastian Meerwein2, Christina Charles-Schoeman3, BERNARD COMBE4, Stephen Hall5, Nasser Khan6, Kyle Carter7, Heidi Camp8 and Andrea Rubbert-Roth9, 1Metroplex Clinical Research Center and University of Texas Southwestern Medical Center, Dallas, TX, 2AbbVie Deutschland GmbH & Co KG, LUDWIGSHAFEN, Germany, 3Division of Rheumatology, University of California, Los Angeles, Santa Monica, CA, 4Montpellier University, Montpellier, France, 5Emeritus Research and Monash University, Melbourne, Australia, 6AbbVie, Inc., Abbott Park, IL, 7AbbVie, Inc., North Chicago, IL, 8Abbvie, Winnetka, IL, 9Division of Rheumatology, Cantonal Clinic St Gallen, St.Gallen, Switzerland
Background/Purpose: To evaluate the long-term efficacy and safety of upadacitinib (UPA) over 5 yrs among patients with rheumatoid arthritis (RA) in a long-term extension (LTE) of the SELECT-BEYOND study.
Methods: In SELECT-BEYOND (a double-blind, randomized phase 3 trial), patients with an inadequate response or intolerance to ≥1 biologic DMARD(s) received UPA 15 mg or 30 mg once daily or placebo (PBO) for 12 wks, each with background conventional synthetic DMARD(s) (csDMARD).1 From wk 12 onwards, patients randomized to PBO switched to UPA 15 mg or 30 mg in a prespecified manner. All patients who completed the wk 24 visit could enter an LTE of up to a total of 5 yrs. Per protocol amendment, patients receiving UPA 30 mg switched to the approved 15 mg dose, with the earliest switch occurring at wk 180. Efficacy data up to wk 260 are shown for patients randomized to UPA 15 mg or 30 mg and reported as observed (AO); missing data for categorical endpoints were also imputed using NRI. Treatment-emergent adverse events (TEAEs) per 100 patient yrs (PY) were summarized over 5 yrs for UPA 15 mg and 30 mg and shown separately for those who switched from UPA 30 mg to 15 mg.
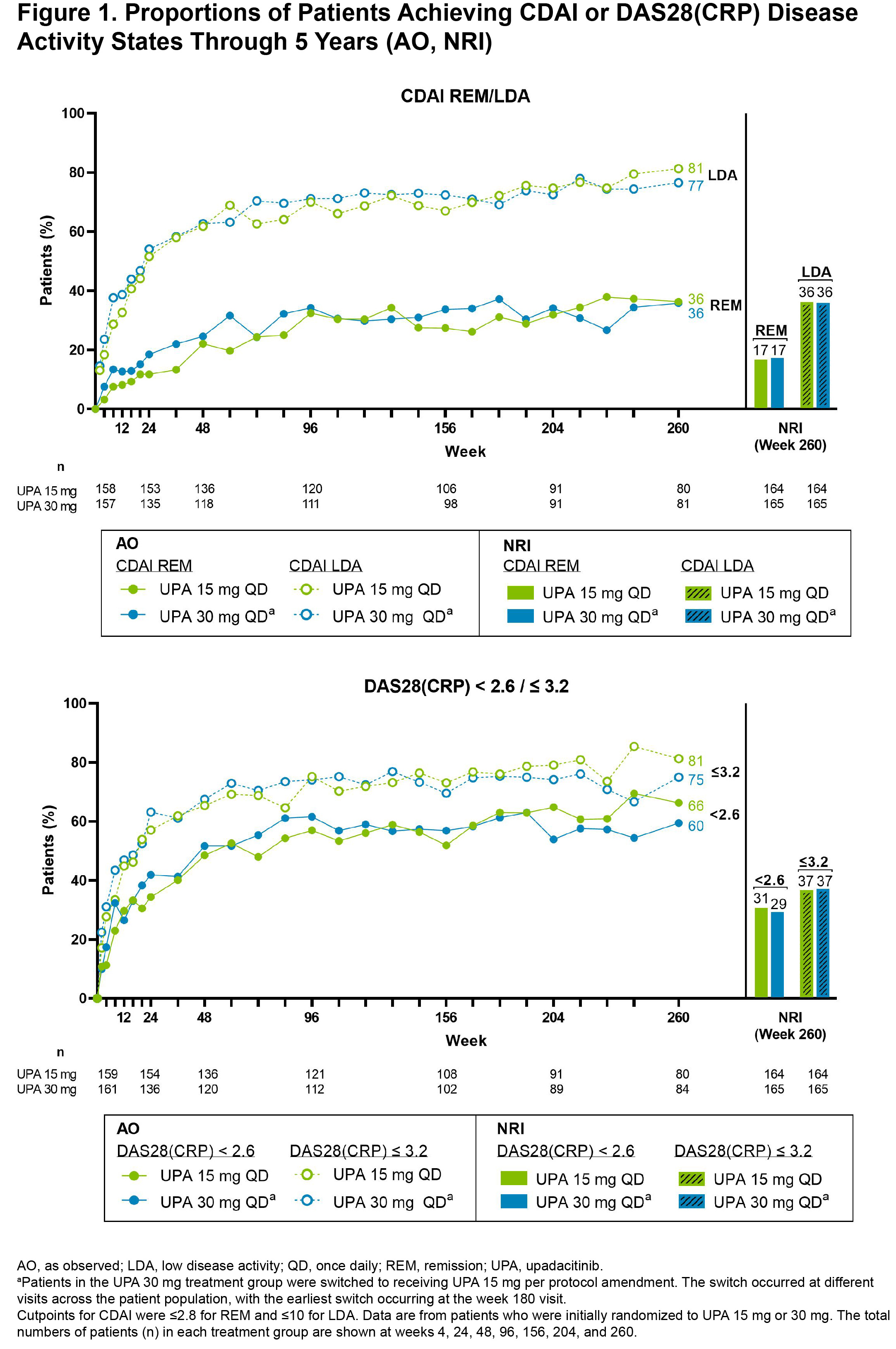
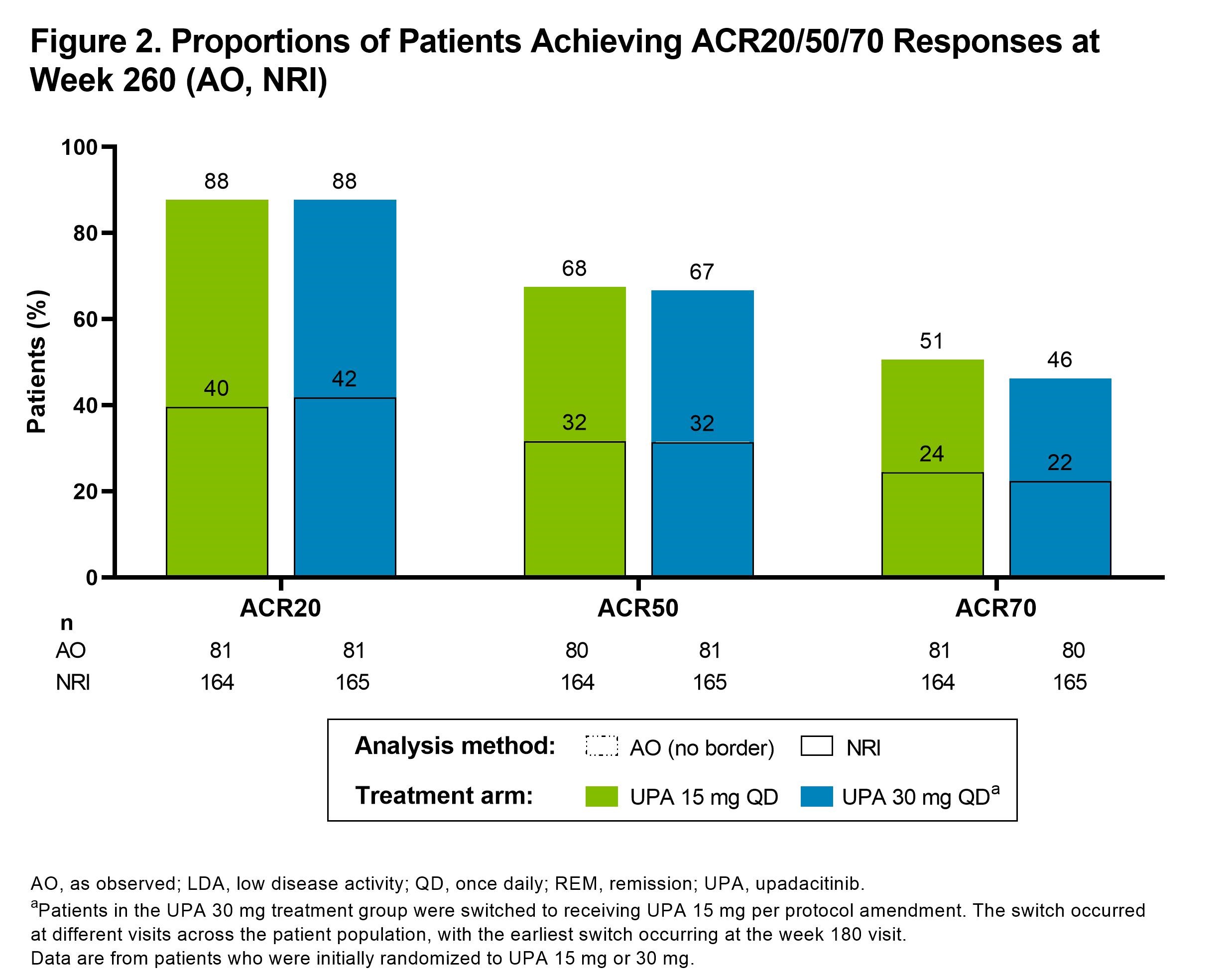
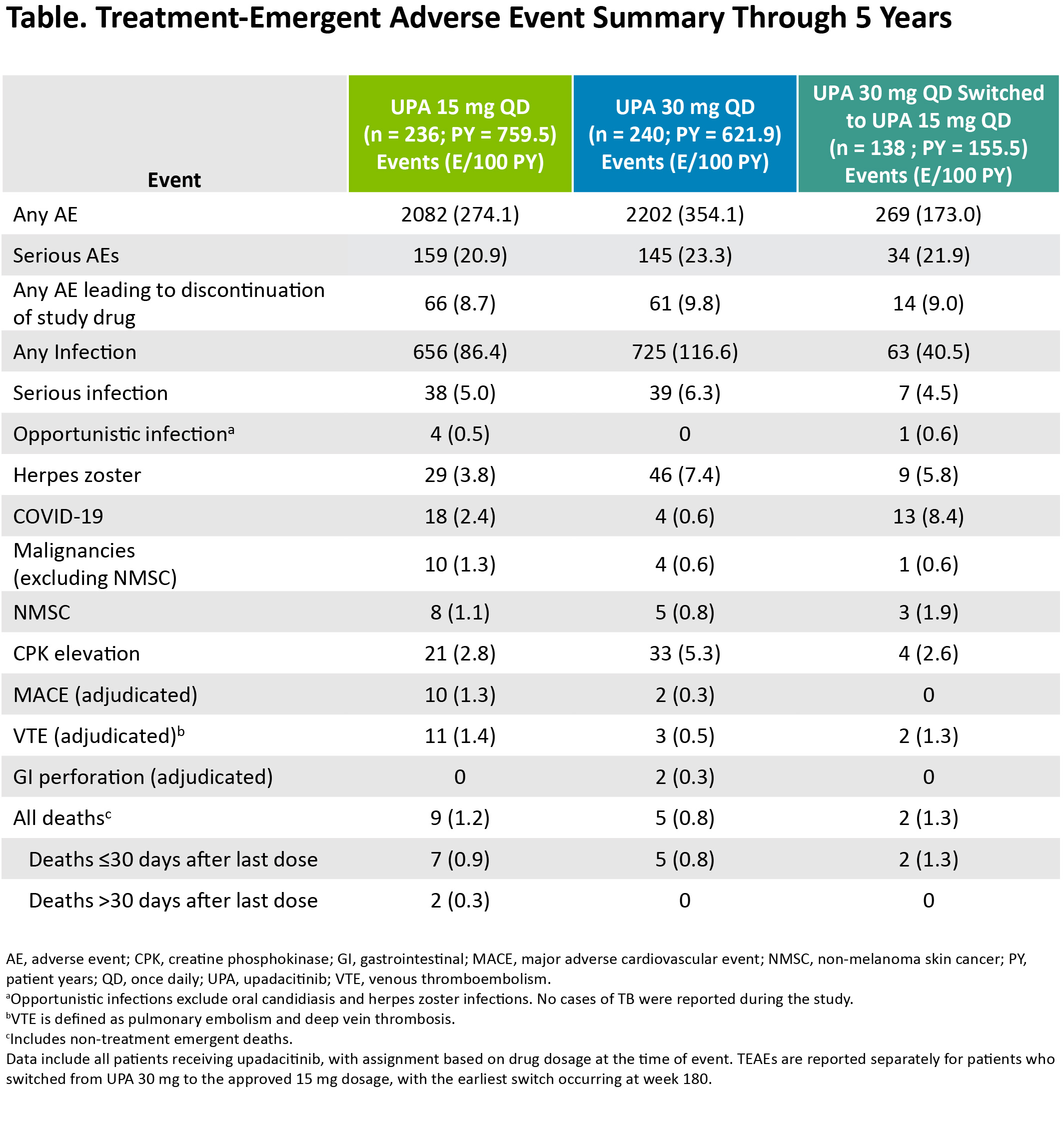
Results: Of the 498 patients randomized, 418 (84%) completed wk 24 and entered the LTE. During the LTE, 197 (40%) patients discontinued study drug due to the following: TEAEs (13%), withdrawal of consent (7%), lack of efficacy (6%), lost to follow-up (4%), or other reasons (11%). Of the patients receiving concomitant corticosteroid or csDMARDs at baseline, approximately 20% and 10%, respectively, discontinued their use during the study. In both UPA dosage groups, patients demonstrated similar clinical improvements over 5 yrs (Figure 1). At wk 260, for example, 36%/36% and 81%/77% of patients receiving UPA 15 mg/30 mg achieved CDAI remission or low disease activity (LDA), respectively (AO). By wk 260, 88%/68%/51% of patients achieved ACR20/50/70 responses on UPA 15 mg and 88%/67%/46% on UPA 30 mg (AO) (Figure 2). Consistent results were observed based on more conservative estimates using NRI. Boolean remission was achieved by 28% of patients on UPA 15 mg and 23% on UPA 30 mg (AO). Functional and pain-related outcomes demonstrated similar improvements with UPA 15 mg/30 mg: the mean change from baseline was -0.6/-0.6 for HAQ-DI and -39/-37 mm for patient's assessment of pain (AO). Patients who switched from PBO showed similar efficacy at wk 260 (data not shown). Also, no apparent loss of benefit was observed with UPA 15 mg treatment in patients who switched from UPA 30 mg to UPA 15 mg. Dose-dependent increases in rates of herpes zoster and CPK elevation were observed with UPA 30 mg vs 15 mg (Table). Treatment-emergent deaths were comparable between both UPA doses. No new safety issues were identified from the long-term study.
Conclusion: Treatment with either UPA 15 mg or 30 mg continued to be effective in improving clinical and functional outcomes in RA. Notably, at wk 260, over three-quarters of patients attained CDAI LDA, a reasonable target for this treatment-refractory population. Overall, the safety profile observed over 5 yrs was consistent with earlier assessments of UPA treatment in this population.2
References
1. Genovese, et al. Lancet 2018;391:2513–24.
2. Genovese, et al. Ann Rheum Dis 2019;78:360–1.
Disclosures: R. Fleischmann, AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galvani, GlaxoSmithKlein (GSK), Janssen, Pfizer, Gilead, UCB, Novartis, Arthrosi, AstraZeneca, Flexion, Genentech, Horizon, Selecta, Viela, Vorso, Vyne; S. Meerwein, AbbVie; C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; B. COMBE, AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, MSD, Pfizer, Roche-Chugai, Novartis; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; N. Khan, AbbVie; K. Carter, AbbVie/Abbott; H. Camp, AbbVie; A. Rubbert-Roth, None.
Background/Purpose: To evaluate the long-term efficacy and safety of upadacitinib (UPA) over 5 yrs among patients with rheumatoid arthritis (RA) in a long-term extension (LTE) of the SELECT-BEYOND study.
Methods: In SELECT-BEYOND (a double-blind, randomized phase 3 trial), patients with an inadequate response or intolerance to ≥1 biologic DMARD(s) received UPA 15 mg or 30 mg once daily or placebo (PBO) for 12 wks, each with background conventional synthetic DMARD(s) (csDMARD).1 From wk 12 onwards, patients randomized to PBO switched to UPA 15 mg or 30 mg in a prespecified manner. All patients who completed the wk 24 visit could enter an LTE of up to a total of 5 yrs. Per protocol amendment, patients receiving UPA 30 mg switched to the approved 15 mg dose, with the earliest switch occurring at wk 180. Efficacy data up to wk 260 are shown for patients randomized to UPA 15 mg or 30 mg and reported as observed (AO); missing data for categorical endpoints were also imputed using NRI. Treatment-emergent adverse events (TEAEs) per 100 patient yrs (PY) were summarized over 5 yrs for UPA 15 mg and 30 mg and shown separately for those who switched from UPA 30 mg to 15 mg.
Results: Of the 498 patients randomized, 418 (84%) completed wk 24 and entered the LTE. During the LTE, 197 (40%) patients discontinued study drug due to the following: TEAEs (13%), withdrawal of consent (7%), lack of efficacy (6%), lost to follow-up (4%), or other reasons (11%). Of the patients receiving concomitant corticosteroid or csDMARDs at baseline, approximately 20% and 10%, respectively, discontinued their use during the study. In both UPA dosage groups, patients demonstrated similar clinical improvements over 5 yrs (Figure 1). At wk 260, for example, 36%/36% and 81%/77% of patients receiving UPA 15 mg/30 mg achieved CDAI remission or low disease activity (LDA), respectively (AO). By wk 260, 88%/68%/51% of patients achieved ACR20/50/70 responses on UPA 15 mg and 88%/67%/46% on UPA 30 mg (AO) (Figure 2). Consistent results were observed based on more conservative estimates using NRI. Boolean remission was achieved by 28% of patients on UPA 15 mg and 23% on UPA 30 mg (AO). Functional and pain-related outcomes demonstrated similar improvements with UPA 15 mg/30 mg: the mean change from baseline was -0.6/-0.6 for HAQ-DI and -39/-37 mm for patient's assessment of pain (AO). Patients who switched from PBO showed similar efficacy at wk 260 (data not shown). Also, no apparent loss of benefit was observed with UPA 15 mg treatment in patients who switched from UPA 30 mg to UPA 15 mg. Dose-dependent increases in rates of herpes zoster and CPK elevation were observed with UPA 30 mg vs 15 mg (Table). Treatment-emergent deaths were comparable between both UPA doses. No new safety issues were identified from the long-term study.
Conclusion: Treatment with either UPA 15 mg or 30 mg continued to be effective in improving clinical and functional outcomes in RA. Notably, at wk 260, over three-quarters of patients attained CDAI LDA, a reasonable target for this treatment-refractory population. Overall, the safety profile observed over 5 yrs was consistent with earlier assessments of UPA treatment in this population.2
References
1. Genovese, et al. Lancet 2018;391:2513–24.
2. Genovese, et al. Ann Rheum Dis 2019;78:360–1.



Disclosures: R. Fleischmann, AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galvani, GlaxoSmithKlein (GSK), Janssen, Pfizer, Gilead, UCB, Novartis, Arthrosi, AstraZeneca, Flexion, Genentech, Horizon, Selecta, Viela, Vorso, Vyne; S. Meerwein, AbbVie; C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; B. COMBE, AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, MSD, Pfizer, Roche-Chugai, Novartis; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; N. Khan, AbbVie; K. Carter, AbbVie/Abbott; H. Camp, AbbVie; A. Rubbert-Roth, None.

