Back
Poster Session A
Vasculitis
Session: (0458–0497) Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
0471: Serum Proteomics in Giant Cell Arteritis: Findings of the Giant Cell Arteritis Treatment with Ultra-short Glucocorticoids and Tocilizumab Trial (The GUSTO Trial)
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LC
Lisa Christ, MD
Inselspital, University Bern, Switzerland
Bern, Switzerland
Abstract Poster Presenter(s)
Lisa Christ1, Andrea Gloor2, Florian Kollert1, Timo Gaber3, Frank Buttgereit3, Stephan Reichenbach4 and Peter Villiger5, 1Department of Rheumatology and Immunology, University of Bern, Inselspital, Bern, Switzerland, 2Department of Internal Medicine, University of Bern, Inselspital, Bern, Switzerland, 3Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin / DRFZ Berlin, Berlin, Germany, 4University of Bern, Institute for Social and Preventive Medicine, Bern, Switzerland, 5Medical Center Monbijou, Rheumatology and Immunology, Bern, Switzerland
Background/Purpose: Measuring disease activity in patients with giant cell arteritis (GCA) remains challenging since appropriate biomarkers are missing, particularly, if tocilizumab (TCZ) is prescribed which blunts the acute phase response. Serum proteome analysis facilitates the identification of biomarkers and allows the inference of pathophysiological and therapeutic changes.
This study aimed (i) to investigate the differential effects of pulse GC-treatment and of ensuing long-term TCZ monotherapy on serum proteins in GCA, (ii) to characterize active disease versus lasting remission, and (iii) to identify proteins potentially serving to monitor disease-activity.
Methods: Eighteen patients with newly diagnosed GCA received 500 mg methylprednisolone intravenously for 3 consecutive days [1]. Thereafter, GC treatment was discontinued and a single dose of TCZ was administered intravenously, followed by weekly subcutaneous TCZ injections from day 10 until week 52. Serum samples were collected before treatment (day 0), on day 3 (after GC treatment), on day 10 and at weeks 4, 24, and 52. Sera were analyzed on Olink® Explore 1536, capturing over 1400 proteins. Protein wise t-tests (threshold, adjusted p-value < 0.05) with adjustment for multiple testing (Benjamini-Hochberg) were performed to identify differentially expressed proteins (DEPs) between day 0 vs day 3 and day 0 vs week 52. DEPs were grouped in pathways identified in the KEGG database.
Results: In total, 86 serum samples from 16 patients were analyzed. Successful treatment resulted in the upregulation of 213 proteins and the downregulation of 221 proteins from day 0 to week 52. The majority of changes occurred within 10 days after treatment start. From week 24 to 52, during established full remission, the protein profile remained unchanged. Forty-nine proteins were inversely regulated by GC and TCZ.
In response to GC pulse therapy, the 10 most significant DEPs included ANGPTL7, AREG, IL1RL1, MATN3, MMP3, SPARCL1 (upregulated) and IL12A, IL12B, KLK10, SIGLEC6 (downregulated).
DEPs mapping to KEGG pathways of interest in GCA (day 0 vs day 3 and day 0 vs week 52, respectively) included: cytokine-cytokine receptor interaction, the PI3K-AKT, JAK-STAT, IL17, TNF, NF-kappa B, Toll-like receptor and MAPK signaling pathways.
Conclusion: The data unveil the biologic effects of GC. Several proteins having been erroneously proposed as disease-activity markers (e.g. MMP3) were found to be induced by GC. TC and GC show in part contrasting effects on protein expression. However, both, GC and TCZ showed effects on macrophage cytokines, such as CCL7, CCL18, or CSF1.
References
1. Christ, et al. Lancet Rheumatol, 2021
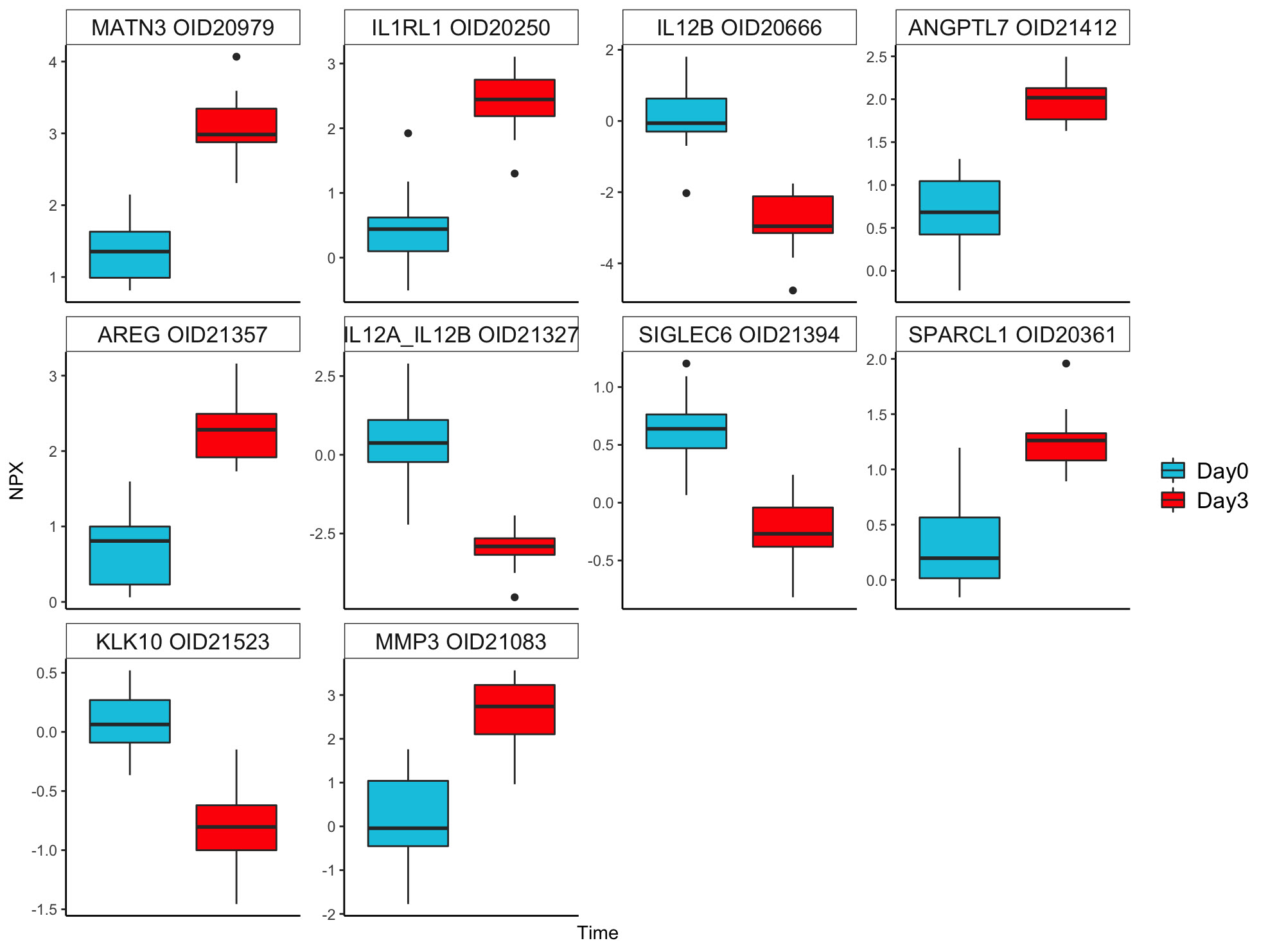 Ten most significant differentially expressed proteins between day 0 vs day 3.
Ten most significant differentially expressed proteins between day 0 vs day 3.
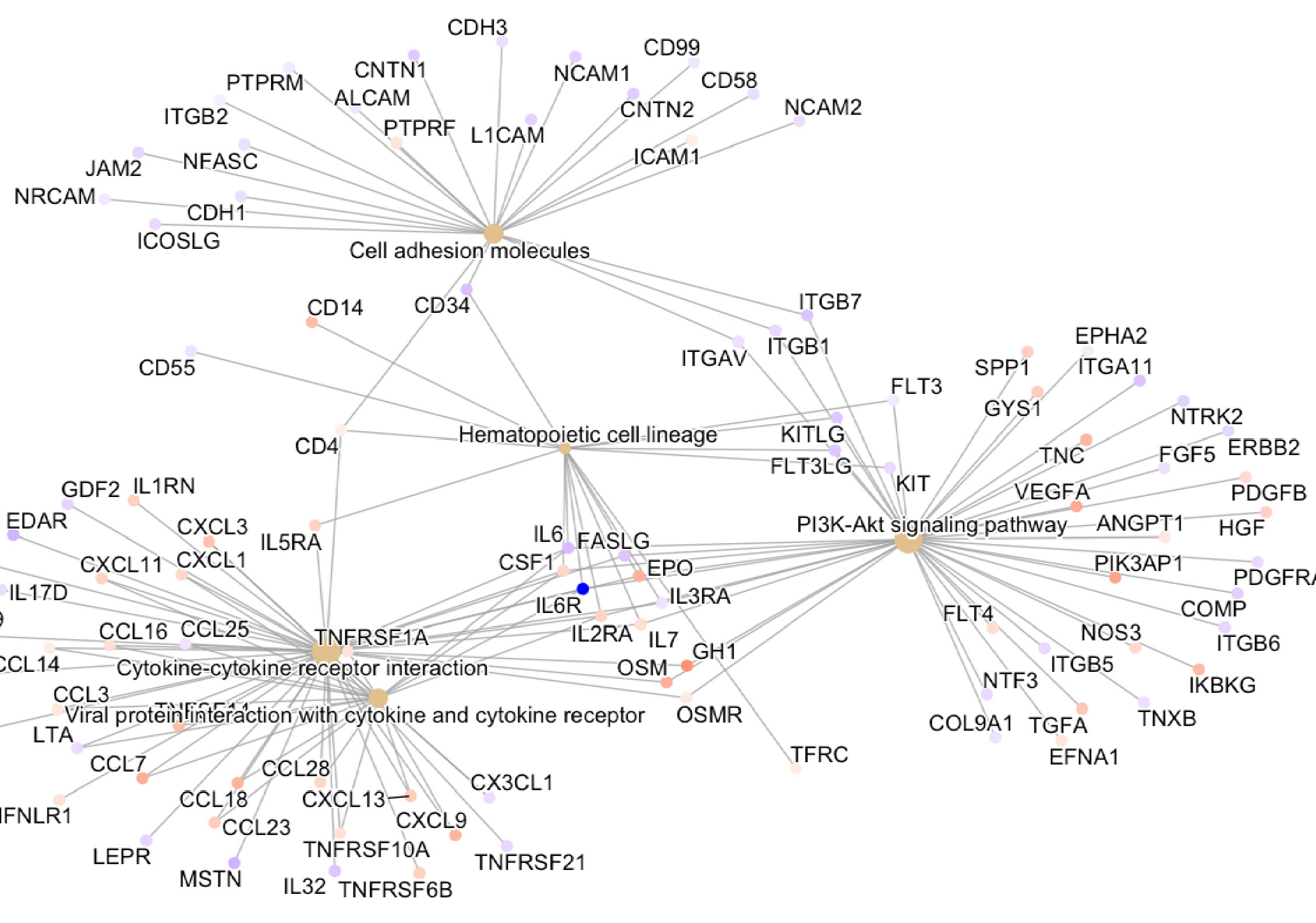 Network plot of the top 5 KEGG pathways from day 0 vs week 52. Size in the figure legend refers to the number of proteins in each pathway. Estimate in the figure legend indicates the log2 fold change from day 0 vs week 52.
Network plot of the top 5 KEGG pathways from day 0 vs week 52. Size in the figure legend refers to the number of proteins in each pathway. Estimate in the figure legend indicates the log2 fold change from day 0 vs week 52.
Disclosures: L. Christ, Novartis, Gilead, Roche, Pfizer, Bristol-Myers Squibb(BMS); A. Gloor, None; F. Kollert, Roche; T. Gaber, None; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer; S. Reichenbach, None; P. Villiger, Roche, Merck/MSD, AbbVie/Abbott, Pfizer, Novartis, Amgen, Bristol-Myers Squibb(BMS).
Background/Purpose: Measuring disease activity in patients with giant cell arteritis (GCA) remains challenging since appropriate biomarkers are missing, particularly, if tocilizumab (TCZ) is prescribed which blunts the acute phase response. Serum proteome analysis facilitates the identification of biomarkers and allows the inference of pathophysiological and therapeutic changes.
This study aimed (i) to investigate the differential effects of pulse GC-treatment and of ensuing long-term TCZ monotherapy on serum proteins in GCA, (ii) to characterize active disease versus lasting remission, and (iii) to identify proteins potentially serving to monitor disease-activity.
Methods: Eighteen patients with newly diagnosed GCA received 500 mg methylprednisolone intravenously for 3 consecutive days [1]. Thereafter, GC treatment was discontinued and a single dose of TCZ was administered intravenously, followed by weekly subcutaneous TCZ injections from day 10 until week 52. Serum samples were collected before treatment (day 0), on day 3 (after GC treatment), on day 10 and at weeks 4, 24, and 52. Sera were analyzed on Olink® Explore 1536, capturing over 1400 proteins. Protein wise t-tests (threshold, adjusted p-value < 0.05) with adjustment for multiple testing (Benjamini-Hochberg) were performed to identify differentially expressed proteins (DEPs) between day 0 vs day 3 and day 0 vs week 52. DEPs were grouped in pathways identified in the KEGG database.
Results: In total, 86 serum samples from 16 patients were analyzed. Successful treatment resulted in the upregulation of 213 proteins and the downregulation of 221 proteins from day 0 to week 52. The majority of changes occurred within 10 days after treatment start. From week 24 to 52, during established full remission, the protein profile remained unchanged. Forty-nine proteins were inversely regulated by GC and TCZ.
In response to GC pulse therapy, the 10 most significant DEPs included ANGPTL7, AREG, IL1RL1, MATN3, MMP3, SPARCL1 (upregulated) and IL12A, IL12B, KLK10, SIGLEC6 (downregulated).
DEPs mapping to KEGG pathways of interest in GCA (day 0 vs day 3 and day 0 vs week 52, respectively) included: cytokine-cytokine receptor interaction, the PI3K-AKT, JAK-STAT, IL17, TNF, NF-kappa B, Toll-like receptor and MAPK signaling pathways.
Conclusion: The data unveil the biologic effects of GC. Several proteins having been erroneously proposed as disease-activity markers (e.g. MMP3) were found to be induced by GC. TC and GC show in part contrasting effects on protein expression. However, both, GC and TCZ showed effects on macrophage cytokines, such as CCL7, CCL18, or CSF1.
References
1. Christ, et al. Lancet Rheumatol, 2021
 Ten most significant differentially expressed proteins between day 0 vs day 3.
Ten most significant differentially expressed proteins between day 0 vs day 3. Network plot of the top 5 KEGG pathways from day 0 vs week 52. Size in the figure legend refers to the number of proteins in each pathway. Estimate in the figure legend indicates the log2 fold change from day 0 vs week 52.
Network plot of the top 5 KEGG pathways from day 0 vs week 52. Size in the figure legend refers to the number of proteins in each pathway. Estimate in the figure legend indicates the log2 fold change from day 0 vs week 52.Disclosures: L. Christ, Novartis, Gilead, Roche, Pfizer, Bristol-Myers Squibb(BMS); A. Gloor, None; F. Kollert, Roche; T. Gaber, None; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer; S. Reichenbach, None; P. Villiger, Roche, Merck/MSD, AbbVie/Abbott, Pfizer, Novartis, Amgen, Bristol-Myers Squibb(BMS).

