Back
Poster Session A
Epidemiology, health policy and outcomes
Session: (0056–0084) Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
0079: Treatment Persistence in First-Line Biologic and Targeted Synthetic DMARD Users with Dual-Seropositive RA in the Medicare Population
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- HZ
Hanke Zheng
Bristol Myers Squibb
Lawrenceville, NJ, United States
Abstract Poster Presenter(s)
Jeffrey Sparks1, Hanke Zheng2, Taylor Schwartz3, Sang Hee Park2, Kris Norris3, Vardhaman Patel4, Scott Robinson3, Keith Wittstock4, Vadim Khaychuk5 and Alison Silverstein3, 1Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 2Bristol Myers Squibb, Princeton, NJ, 3Inovalon Insights, Bowie, MD, 4Bristol Myers Squibb, Lawrence Township, NJ, 5Bristol Myers Squibb, Pennington, MA
Background/Purpose: The objective of this study was to compare persistence among first-line (1L) abatacept, TNF inhibitors (TNFi), and Janus kinase inhibitors (JAKi) in patients with dual seropositive (anti-CCP+/RF+) RA enrolled in Medicare Fee-For-Service (FFS).
Methods: This retrospective cohort study used 100% Medicare FFS claims (Parts A/B/D) linked to Prognos (New York, NY) laboratory data from January 1, 2012 to December 31, 2019. Beneficiaries were included if they had ≥ 2 International Classification of Diseases, Ninth/Tenth diagnosis claims for RA; had ≥1 RF+ and anti-CCP+ test result, and received abatacept, TNFi, or JAKi as 1L treatment (date of treatment initiation was the index date). Patients were required to have continuous enrollment for 6 months before and 12 months after index treatment initiation. Patients were biologic DMARD (bDMARD)- and targeted synthetic DMARD (tsDMARD)-naïve at index treatment initiation. The primary outcome of the study was persistence, defined as absence of gap in therapy > 60 days (≤ 90 days over 12 months) or treatment switch, during follow-up. Additionally, duration of persistence, which was the number of days on therapy prior to discontinuation (presence of gap in therapy > 60 days or treatment switch) or end of follow-up, was assessed. Kaplan–Meier curves were generated to describe the unadjusted time to discontinuation. Hazard ratios (HRs) for discontinuation were derived from a Cox proportional hazard model, adjusting for baseline demographics and clinical characteristics.
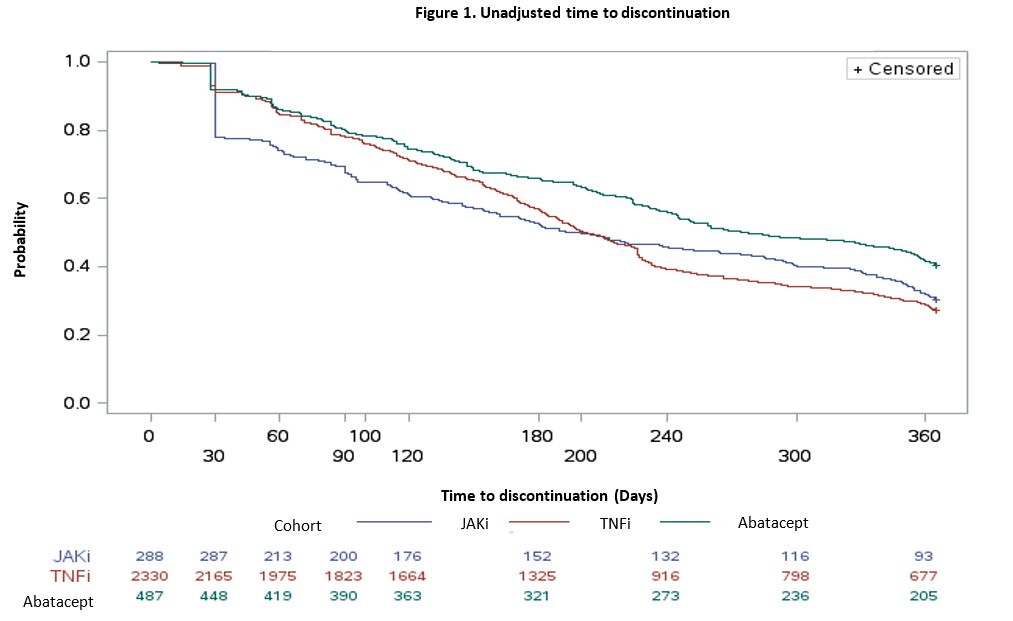
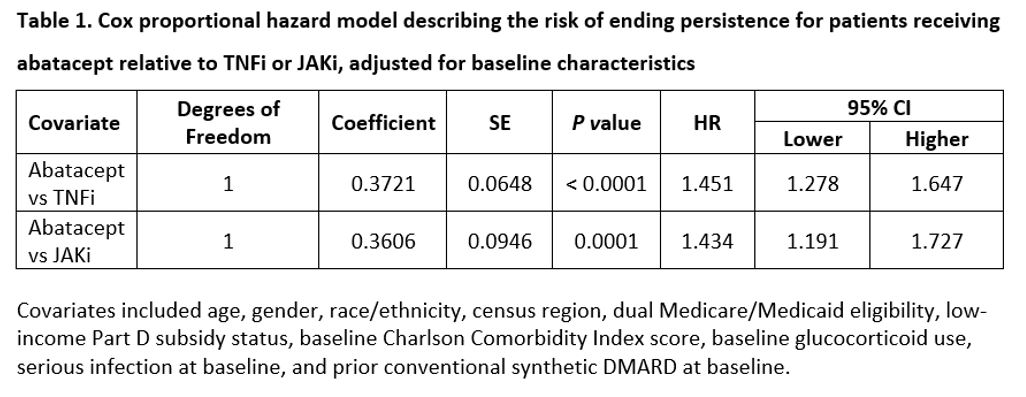
Results: A total of 3,105 bDMARD- and tsDMARD-naïve patients with dual seropositive RA were included in the study (abatacept: 487; TNFi: 2,330; JAKi: 288). Mean age in the abatacept cohort [reference group] (68.8 years) was higher than the TNFi (66.2; p-value < 0.0001) and the JAKi (63.8; p-value < 0.0001) cohorts. The proportion of White patients was highest in the abatacept cohort (77% vs. 74% [TNFi]; p-value=0.2323 vs. 63% [JAKi]; p-value < 0.0001). The proportion of patients with conventional synthetic DMARD fills during the baseline in the reference abatacept group was numerically lower than the JAKi cohort (1.7% vs. 2.2%; p-value=0.7240) but higher than the TNFi cohort (1.7% vs. 0.7%; p-value < 0.05). Abatacept (ref) (48%) had the highest persistence rate at 12 months (48%) compared to TNFi (33%; p-value < 0.0001) and JAKi (39%; p-value < 0.05). The unadjusted median time to discontinuation for patients receiving abatacept was higher (median [interquartile range], 276 [119-365] days) than for those on TNFi (202 [105-365] days) and JAKi (197 [58-365] days) (Figure 1). The adjusted risk of discontinuation was higher in TNFi (HR=1.45, 95% CI: 1.27–1.64) and JAKi cohorts (HR=1.43, 95% CI:1.19–1.72) compared with the abatacept cohort (Table 1).
Conclusion: In bDMARD and tsDMARD-naïve Medicare beneficiaries with dual-seropositive RA, abatacept users had the highest persistence rate at 12 months and median time to discontinuation compared with the TNFi and JAKi cohorts. Future research is needed to understand the reasons for treatment discontinuation.
Editorial support: Sarah Millard (Caudex), funded by Bristol Myers Squibb.
Disclosures: J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; H. Zheng, Bristol Myers Squibb; T. Schwartz, Bristol Myers Squibb; S. Park, Bristol Myers Squibb; K. Norris, None; V. Patel, Bristol Myers Squibb; S. Robinson, Bristol Myers Squibb; K. Wittstock, Bristol Myers Squibb; V. Khaychuk, Bristol Myers Squibb; A. Silverstein, Bristol Myers Squibb.
Background/Purpose: The objective of this study was to compare persistence among first-line (1L) abatacept, TNF inhibitors (TNFi), and Janus kinase inhibitors (JAKi) in patients with dual seropositive (anti-CCP+/RF+) RA enrolled in Medicare Fee-For-Service (FFS).
Methods: This retrospective cohort study used 100% Medicare FFS claims (Parts A/B/D) linked to Prognos (New York, NY) laboratory data from January 1, 2012 to December 31, 2019. Beneficiaries were included if they had ≥ 2 International Classification of Diseases, Ninth/Tenth diagnosis claims for RA; had ≥1 RF+ and anti-CCP+ test result, and received abatacept, TNFi, or JAKi as 1L treatment (date of treatment initiation was the index date). Patients were required to have continuous enrollment for 6 months before and 12 months after index treatment initiation. Patients were biologic DMARD (bDMARD)- and targeted synthetic DMARD (tsDMARD)-naïve at index treatment initiation. The primary outcome of the study was persistence, defined as absence of gap in therapy > 60 days (≤ 90 days over 12 months) or treatment switch, during follow-up. Additionally, duration of persistence, which was the number of days on therapy prior to discontinuation (presence of gap in therapy > 60 days or treatment switch) or end of follow-up, was assessed. Kaplan–Meier curves were generated to describe the unadjusted time to discontinuation. Hazard ratios (HRs) for discontinuation were derived from a Cox proportional hazard model, adjusting for baseline demographics and clinical characteristics.
Results: A total of 3,105 bDMARD- and tsDMARD-naïve patients with dual seropositive RA were included in the study (abatacept: 487; TNFi: 2,330; JAKi: 288). Mean age in the abatacept cohort [reference group] (68.8 years) was higher than the TNFi (66.2; p-value < 0.0001) and the JAKi (63.8; p-value < 0.0001) cohorts. The proportion of White patients was highest in the abatacept cohort (77% vs. 74% [TNFi]; p-value=0.2323 vs. 63% [JAKi]; p-value < 0.0001). The proportion of patients with conventional synthetic DMARD fills during the baseline in the reference abatacept group was numerically lower than the JAKi cohort (1.7% vs. 2.2%; p-value=0.7240) but higher than the TNFi cohort (1.7% vs. 0.7%; p-value < 0.05). Abatacept (ref) (48%) had the highest persistence rate at 12 months (48%) compared to TNFi (33%; p-value < 0.0001) and JAKi (39%; p-value < 0.05). The unadjusted median time to discontinuation for patients receiving abatacept was higher (median [interquartile range], 276 [119-365] days) than for those on TNFi (202 [105-365] days) and JAKi (197 [58-365] days) (Figure 1). The adjusted risk of discontinuation was higher in TNFi (HR=1.45, 95% CI: 1.27–1.64) and JAKi cohorts (HR=1.43, 95% CI:1.19–1.72) compared with the abatacept cohort (Table 1).
Conclusion: In bDMARD and tsDMARD-naïve Medicare beneficiaries with dual-seropositive RA, abatacept users had the highest persistence rate at 12 months and median time to discontinuation compared with the TNFi and JAKi cohorts. Future research is needed to understand the reasons for treatment discontinuation.
Editorial support: Sarah Millard (Caudex), funded by Bristol Myers Squibb.


Disclosures: J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; H. Zheng, Bristol Myers Squibb; T. Schwartz, Bristol Myers Squibb; S. Park, Bristol Myers Squibb; K. Norris, None; V. Patel, Bristol Myers Squibb; S. Robinson, Bristol Myers Squibb; K. Wittstock, Bristol Myers Squibb; V. Khaychuk, Bristol Myers Squibb; A. Silverstein, Bristol Myers Squibb.

