Back
Poster Session A
Vasculitis
Session: (0458–0497) Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
0474: Utility of Optimization of Tocilizumab Therapy in Giant Cell Arteritis: A Multicenter Study of 471 Patients
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- FB
Fabricio Benavides, MD
Hospital Universitario Marqués de Valdecilla
Santander, Spain
Abstract Poster Presenter(s)
Fabricio Benavides Villanueva1, Cristina Corrales1, Javier Loricera2, Monica Calderón-Goercke1, Clara Moriano3, Santos Castañeda4, FRANCISCO JAVIER NARVAEZ5, Vicente Aldasoro6, Olga Maiz7, Rafael Melero8, Juan Ignacio Villa9, Paloma Vela-Casampere10, Susana Romero Yuste11, José Luis Callejas12, Eugenio De Miguel13, Eva Galíndez-Agirregoikoa14, Francisca Sivera15, Jesús Carlos Fernández-López16, Carles Galisteo17, Ivan Ferraz Amaro18, JULIO SANCHEZ MARTIN1, Lara Sánchez-Bilbao1, Jose Luis Hernández1, Miguel Angel Gonzalez Gay19 and Ricardo Blanco2, 1Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 3Complejo Asistencial Universitario de León, León, Spain, 4Division of Rheumatology, Hospital Universitario de La Princesa, IIS-Princesa, Madrid, Spain, 5Hospital de Bellvitge, Barcelona, Spain, 6Hospital Universitario de Navarra, Pamplona, Spain, 7Hospital Universitario de Donostia, San Sebastián, Spain, 8Complexo Hospitalario Universitario de Vigo, Vigo, Spain, 9Hospital Sierrallana, Torrelavega, Spain, 10Hospital General Universitario Alicante, Alicante, Spain, 11Complexo Hospitalario Universitario, Pontevedra, Spain, 12Hospital San Cecilio, Granada, Andalucia, Spain, 13Hospital Universitario La Paz, Madrid, Spain, 14Basurto University Hospital, Bilbao, Spain, 15Hospital Universitario de Elda, San Vicente del Raspeig, Spain, 16Complejo H. Universitario de A Coruña, A Coruña, Spain, 17Hospital Parc Tauli,, Sabadel, Spain, 18Division of Rheumatology. Hospital Universitario de Canarias. Spain., Santa Cruz de Tenerife, Spain, 19Hospital Universitario Marques de Valdecilla, Lugo, Spain
Background/Purpose: Tocilizumab (TCZ) has shown to be useful in the treatment of large-vessel vasculitis, including giant cell arteritis (GCA). There is general agreement on the initial and the standard maintenance dose of TCZ. However, information on duration and optimization of TCZ in GCA is really scarce./
Our aim was to assess the effectiveness and safety of TCZ therapy optimization in an unselected wide series of GCA in real-world clinical practice.
Methods: Multicenter study on 471 patients with GCA who received TCZ therapy. Once complete remission was reached (n=231) TCZ was optimized in 125 patients. We compared patients in whom TCZ was optimized (TCZOPT group) or not (TCZNON-OPT group). Complete remission was defined as normalization of clinical and analytical (CRP and ESR) data. Optimization was done by decreasing the dose and/or prolonging the TCZ dosing interval progressively. We performed a comparison in effectiveness and safety parameters between optimized and non-optimized patients.
Results: We evaluated 231 GCA patients treated with TCZ with complete remission. No demographic or laboratory data differences was observed at TCZ onset between both groups (TABLE). The mean prednisone dose was higher in the TCZNON-OPT group at TCZ onset. The first TCZ optimization was performed after a median [25-75th] follow-up of 12 [6-17] months.
The median prednisone dose at first TCZ optimization was 2.5 [0-5] mg/day. At the end of follow-up prolonged remission was observed in 78.2% of TCZOPT group compared with 66.7% in the TCZNON-OPT group (p= 0.001) (FIGURE). Seven (5.6%) of the 125 optimized cases relapsed. Serious adverse events were similar in both groups, while serious infections were more frequent in the TCZNON-OPT group (p=0.009).
Conclusion: Once complete remission is reached in GCA patients under TCZ treatment, optimization of biologic may be performed. Based on our experience it could be performed by reducing the dose or by prolonging dosing interval of TCZ. It seems to be an effective and safe practice.
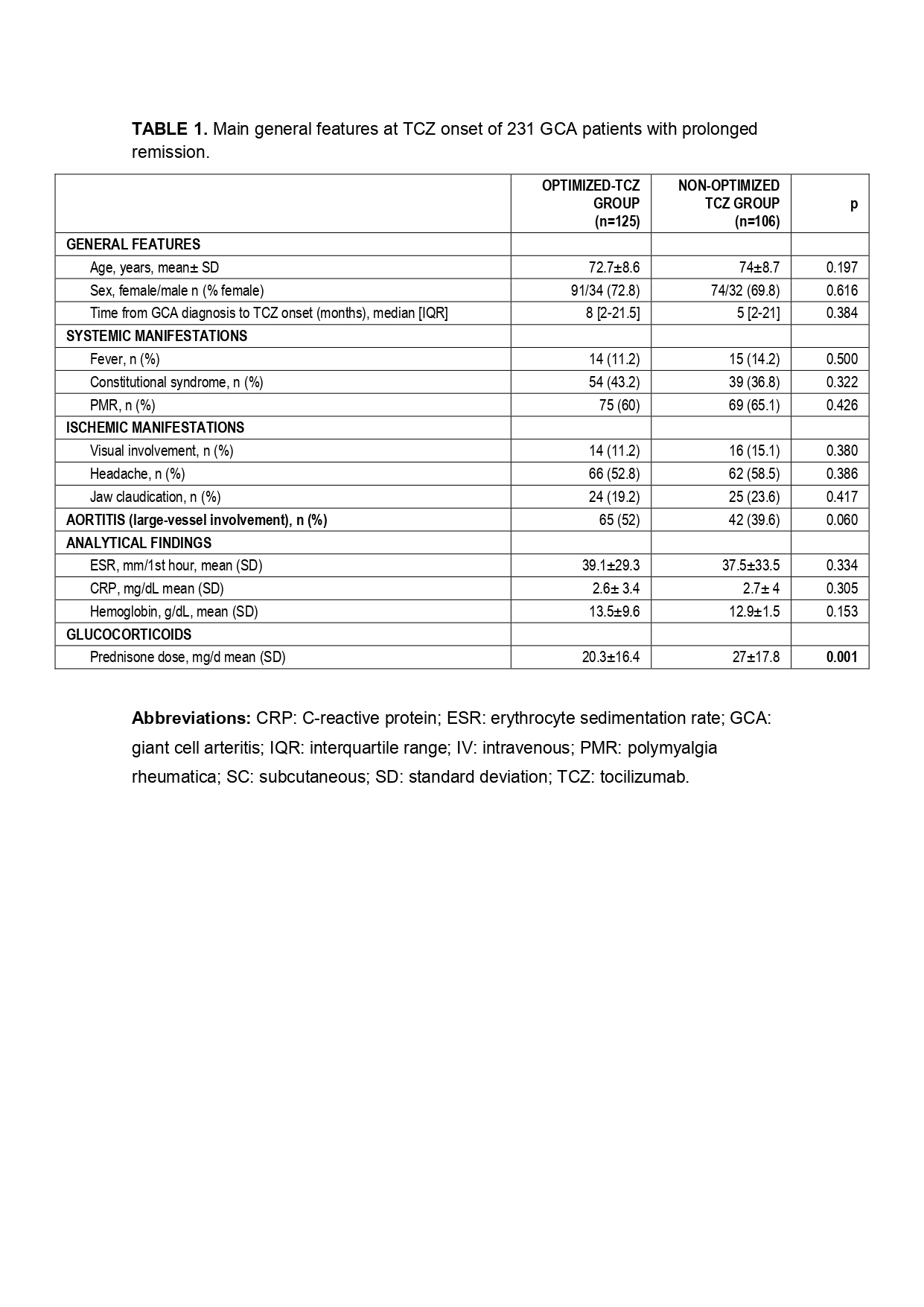 TABLE 1. Main general features at TCZ onset of 231 GCA patients with prolonged remission.
TABLE 1. Main general features at TCZ onset of 231 GCA patients with prolonged remission.
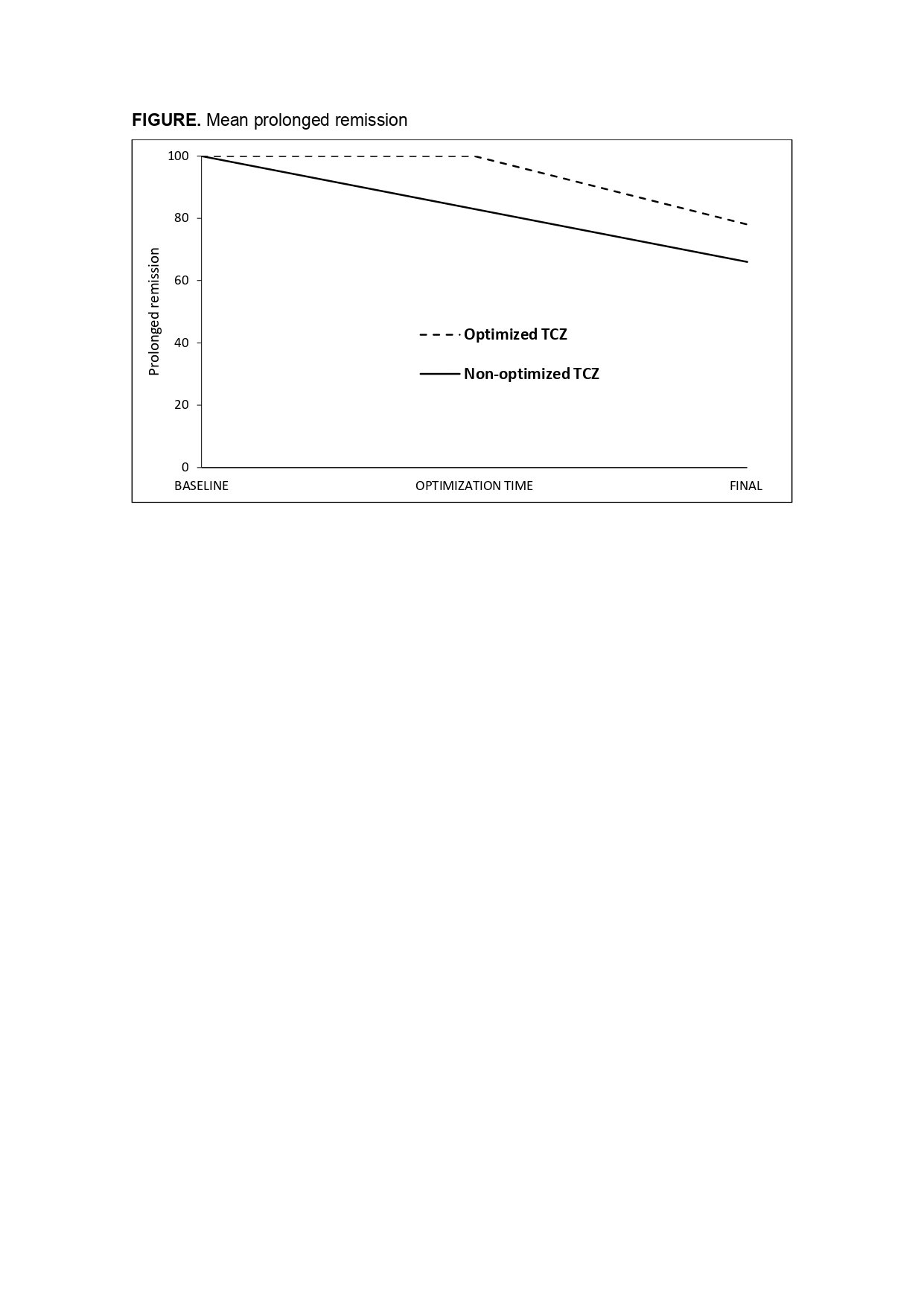 FIGURE. Mean prolonged remission
FIGURE. Mean prolonged remission
Disclosures: F. Benavides Villanueva, None; C. Corrales, None; J. Loricera, Novartis, UCB, Celgene, Roche; M. Calderón-Goercke, None; C. Moriano, None; S. Castañeda, Roche; F. NARVAEZ, None; V. Aldasoro, None; O. Maiz, None; R. Melero, None; J. Villa, None; P. Vela-Casampere, None; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; J. Callejas, None; E. De Miguel, None; E. Galíndez-Agirregoikoa, None; F. Sivera, None; J. Fernández-López, None; C. Galisteo, None; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; J. SANCHEZ MARTIN, None; L. Sánchez-Bilbao, Eli Lilly; J. Hernández, None; M. Gonzalez Gay, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Tocilizumab (TCZ) has shown to be useful in the treatment of large-vessel vasculitis, including giant cell arteritis (GCA). There is general agreement on the initial and the standard maintenance dose of TCZ. However, information on duration and optimization of TCZ in GCA is really scarce./
Our aim was to assess the effectiveness and safety of TCZ therapy optimization in an unselected wide series of GCA in real-world clinical practice.
Methods: Multicenter study on 471 patients with GCA who received TCZ therapy. Once complete remission was reached (n=231) TCZ was optimized in 125 patients. We compared patients in whom TCZ was optimized (TCZOPT group) or not (TCZNON-OPT group). Complete remission was defined as normalization of clinical and analytical (CRP and ESR) data. Optimization was done by decreasing the dose and/or prolonging the TCZ dosing interval progressively. We performed a comparison in effectiveness and safety parameters between optimized and non-optimized patients.
Results: We evaluated 231 GCA patients treated with TCZ with complete remission. No demographic or laboratory data differences was observed at TCZ onset between both groups (TABLE). The mean prednisone dose was higher in the TCZNON-OPT group at TCZ onset. The first TCZ optimization was performed after a median [25-75th] follow-up of 12 [6-17] months.
The median prednisone dose at first TCZ optimization was 2.5 [0-5] mg/day. At the end of follow-up prolonged remission was observed in 78.2% of TCZOPT group compared with 66.7% in the TCZNON-OPT group (p= 0.001) (FIGURE). Seven (5.6%) of the 125 optimized cases relapsed. Serious adverse events were similar in both groups, while serious infections were more frequent in the TCZNON-OPT group (p=0.009).
Conclusion: Once complete remission is reached in GCA patients under TCZ treatment, optimization of biologic may be performed. Based on our experience it could be performed by reducing the dose or by prolonging dosing interval of TCZ. It seems to be an effective and safe practice.
 TABLE 1. Main general features at TCZ onset of 231 GCA patients with prolonged remission.
TABLE 1. Main general features at TCZ onset of 231 GCA patients with prolonged remission.  FIGURE. Mean prolonged remission
FIGURE. Mean prolonged remission Disclosures: F. Benavides Villanueva, None; C. Corrales, None; J. Loricera, Novartis, UCB, Celgene, Roche; M. Calderón-Goercke, None; C. Moriano, None; S. Castañeda, Roche; F. NARVAEZ, None; V. Aldasoro, None; O. Maiz, None; R. Melero, None; J. Villa, None; P. Vela-Casampere, None; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; J. Callejas, None; E. De Miguel, None; E. Galíndez-Agirregoikoa, None; F. Sivera, None; J. Fernández-López, None; C. Galisteo, None; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; J. SANCHEZ MARTIN, None; L. Sánchez-Bilbao, Eli Lilly; J. Hernández, None; M. Gonzalez Gay, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

