Back
Poster Session D
Immunobiology
Session: (1681–1706) Innate Immunity Poster: Basic and Translational Science
1688: Biomechanical Phenotype of Circulating Neutrophils Is Altered in ANCA Associated Vasculitis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NP
Noelle Pisacano, BSc, MSc
Imperial College London
London, United Kingdom
Abstract Poster Presenter(s)
Noelle Pisacano1, Stephen P. McAdoo1, Jochen Guck2, Charles D. Pusey1, Edwin R. Chilvers1, Andrew S. Cowburn1, Katharine M. Lodge1 and Maria F. Prendecki1, 1Imperial College London, London, United Kingdom, 2Max Planck Institute, Erlangen, Germany
Background/Purpose: Real Time-Deformability Cytometry (RT-DC) is a novel technique able to identify morpho-rheological characteristics of individual cells such as size, deformability, and elasticity using only 50µl of whole blood. Cells in suspension flow through a microfluidic channel while hydrodynamic force is applied, leading to reversible deformation of individual cells from shear stress and pressure gradients. Cell brightness and area can be used to identify individual cell types. The morpho-rheological characteristics of immune cells in ANCA associated vasculitis (AAV) are unknown.
Methods: Whole blood from healthy controls (HC), patients with active AAV, or patients with AAV in remission was analysed using RT-DC. The diagnosis of AAV was based upon positive ANCA testing, with either (i) pauci-immune glomerulonephritis, or (ii) typical clinical features of extra-renal AAV. Patients with active disease may have received steroids prior to sampling but those who received other immunosuppression or cytotoxics were excluded. Remission was defined as a BVAS of 0 with prednisolone dose ≤7.5mg/day. Blood was collected into K2 EDTA and diluted 1:20 in 1xPBS/0.6% methylcellulose. Cell suspensions were flowed across a Flic20 polydimethylsiloxane microfluidic chip, containing reservoirs connected by a measurement channel with a 20x20 μm2 cross-section. RT-DC measurements were collected at a cellular flow rate of 0.12μL/s using a high-speed CMOS camera to capture images at a frame rate of 2000 frames/second. ShapeOut software was used to calculate cell size, deformation, and elasticity.
Results: There was no difference in neutrophil size between patients with active AAV (n=15), patients with AAV in remission (n=31), and HC (n=15) (Figure 1A). Neutrophils from patients with active AAV were significantly stiffer than patients in remission or HC, displaying decreased deformation (Figure 1B; median 0.084, 0.085, and 0.078 au respectively, p=0.0068) and increased elasticity (Figure 1C; median 0.973, 0.968 and 1.006 au respectively, p=0.0196). In patients with active AAV, there was strong inverse correlation between neutrophil deformation and disease activity as measured by BVAS score (Figure 1D; r=-0.573, p=0.0328). Neutrophils isolated from healthy donors exhibited a trend towards decreased deformability when primed with TNF and stimulated with MPO-ANCA IgG compared to unstimulated cells or those treated with healthy donor IgG in place of MPO-ANCA IgG (Figure 1E; median 0.086, 0.085, and 0.083 for unstimulated, control IgG, and MPO-ANCA IgG respectively, ns).
Conclusion: Neutrophils from patients display distinct physical properties in active AAV which correlate with disease activity. Similar results are seen when neutrophils are stimulated in vitro with MPO-ANCA IgG. This phenotype of increased cell stiffness may lead to increased neutrophil retention in pulmonary and renal microvasculature, thus increasing the potential for neutrophil-endothelial cell interactions and microvascular damage. Morphorheological parameters can be rapidly measured using a small volume of whole blood; thus RT-DC may be a useful technique to aid identification of disease activity in AAV and to guide treatment.
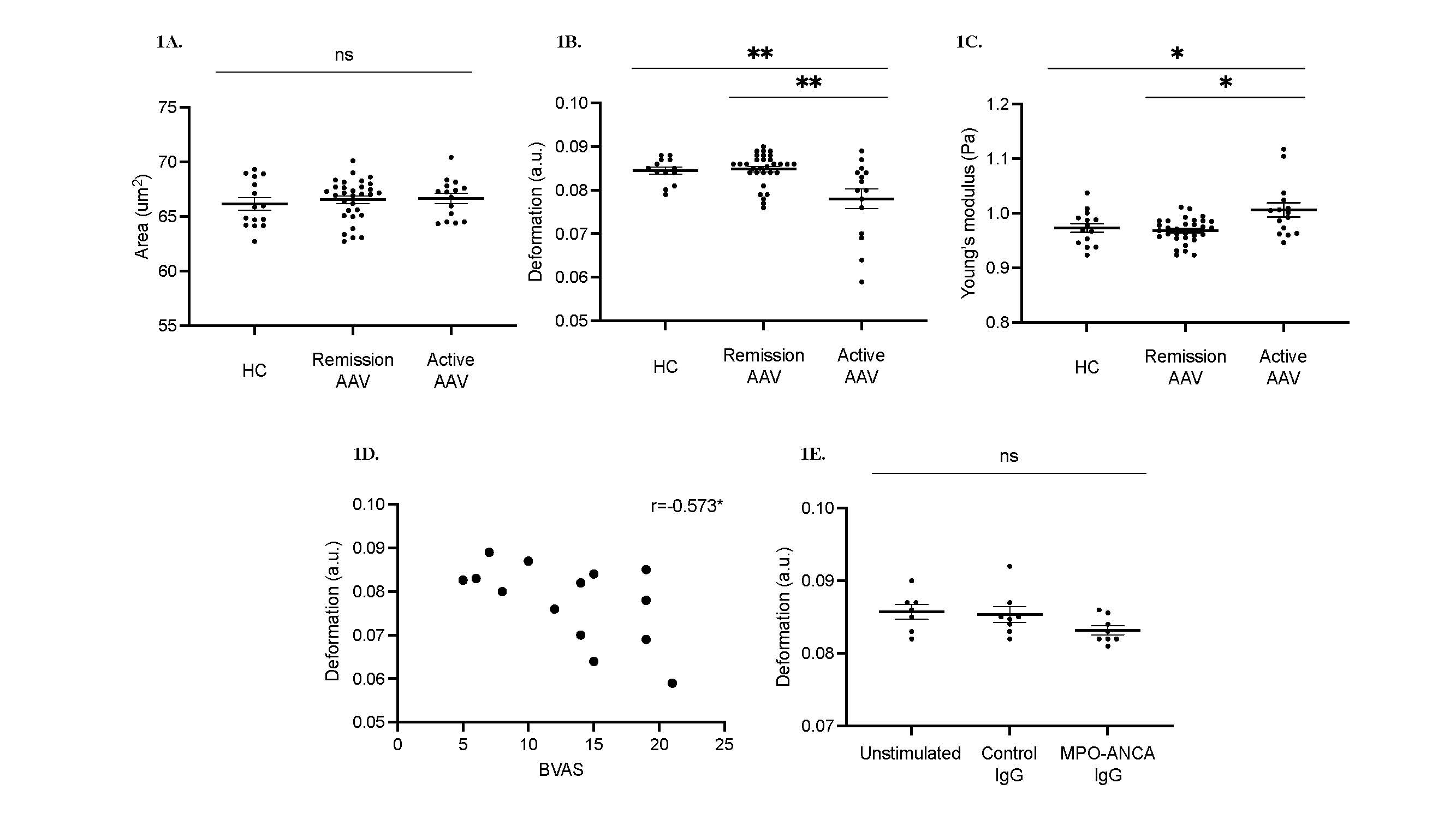 Figure 1. Morpho-rheological properties of circulating neutrophils in AAV
Figure 1. Morpho-rheological properties of circulating neutrophils in AAV
(A)-(C) RTDC profiles of Whole blood from active and remission AAV patients, and HC.
Active AAV (n=15), remission (n=31) and HC (n=15); data represent mean ± SEM A. Size, B. Deformability, and C. Elasticity.
(D) Correlation of deformability and BVAS score.
(E) RTDC profiles of Unstimulated and stimulated neutrophils from HC.
HC neutrophils (2x106) primed with 2 ng/mL of TNF for 15 minutes, treated with 100 ug/mL control or MPO-ANCA IgG for 1 hour (n=8).
Data are shown as median with IQR, statistical comparison by Kruskal-Wallis test, with Dunn’s post-test comparison; *p < 0.05 **p < 0.01, ns= non-significant.
Disclosures: N. Pisacano, None; S. McAdoo, None; J. Guck, None; C. Pusey, None; E. Chilvers, None; A. Cowburn, None; K. Lodge, None; M. Prendecki, None.
Background/Purpose: Real Time-Deformability Cytometry (RT-DC) is a novel technique able to identify morpho-rheological characteristics of individual cells such as size, deformability, and elasticity using only 50µl of whole blood. Cells in suspension flow through a microfluidic channel while hydrodynamic force is applied, leading to reversible deformation of individual cells from shear stress and pressure gradients. Cell brightness and area can be used to identify individual cell types. The morpho-rheological characteristics of immune cells in ANCA associated vasculitis (AAV) are unknown.
Methods: Whole blood from healthy controls (HC), patients with active AAV, or patients with AAV in remission was analysed using RT-DC. The diagnosis of AAV was based upon positive ANCA testing, with either (i) pauci-immune glomerulonephritis, or (ii) typical clinical features of extra-renal AAV. Patients with active disease may have received steroids prior to sampling but those who received other immunosuppression or cytotoxics were excluded. Remission was defined as a BVAS of 0 with prednisolone dose ≤7.5mg/day. Blood was collected into K2 EDTA and diluted 1:20 in 1xPBS/0.6% methylcellulose. Cell suspensions were flowed across a Flic20 polydimethylsiloxane microfluidic chip, containing reservoirs connected by a measurement channel with a 20x20 μm2 cross-section. RT-DC measurements were collected at a cellular flow rate of 0.12μL/s using a high-speed CMOS camera to capture images at a frame rate of 2000 frames/second. ShapeOut software was used to calculate cell size, deformation, and elasticity.
Results: There was no difference in neutrophil size between patients with active AAV (n=15), patients with AAV in remission (n=31), and HC (n=15) (Figure 1A). Neutrophils from patients with active AAV were significantly stiffer than patients in remission or HC, displaying decreased deformation (Figure 1B; median 0.084, 0.085, and 0.078 au respectively, p=0.0068) and increased elasticity (Figure 1C; median 0.973, 0.968 and 1.006 au respectively, p=0.0196). In patients with active AAV, there was strong inverse correlation between neutrophil deformation and disease activity as measured by BVAS score (Figure 1D; r=-0.573, p=0.0328). Neutrophils isolated from healthy donors exhibited a trend towards decreased deformability when primed with TNF and stimulated with MPO-ANCA IgG compared to unstimulated cells or those treated with healthy donor IgG in place of MPO-ANCA IgG (Figure 1E; median 0.086, 0.085, and 0.083 for unstimulated, control IgG, and MPO-ANCA IgG respectively, ns).
Conclusion: Neutrophils from patients display distinct physical properties in active AAV which correlate with disease activity. Similar results are seen when neutrophils are stimulated in vitro with MPO-ANCA IgG. This phenotype of increased cell stiffness may lead to increased neutrophil retention in pulmonary and renal microvasculature, thus increasing the potential for neutrophil-endothelial cell interactions and microvascular damage. Morphorheological parameters can be rapidly measured using a small volume of whole blood; thus RT-DC may be a useful technique to aid identification of disease activity in AAV and to guide treatment.
 Figure 1. Morpho-rheological properties of circulating neutrophils in AAV
Figure 1. Morpho-rheological properties of circulating neutrophils in AAV(A)-(C) RTDC profiles of Whole blood from active and remission AAV patients, and HC.
Active AAV (n=15), remission (n=31) and HC (n=15); data represent mean ± SEM A. Size, B. Deformability, and C. Elasticity.
(D) Correlation of deformability and BVAS score.
(E) RTDC profiles of Unstimulated and stimulated neutrophils from HC.
HC neutrophils (2x106) primed with 2 ng/mL of TNF for 15 minutes, treated with 100 ug/mL control or MPO-ANCA IgG for 1 hour (n=8).
Data are shown as median with IQR, statistical comparison by Kruskal-Wallis test, with Dunn’s post-test comparison; *p < 0.05 **p < 0.01, ns= non-significant.
Disclosures: N. Pisacano, None; S. McAdoo, None; J. Guck, None; C. Pusey, None; E. Chilvers, None; A. Cowburn, None; K. Lodge, None; M. Prendecki, None.

