Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0420: What Does It Mean - A Good Response to NSAIDs? A Systematic Comparison of Patients with Axial Spondyloarthritis and Controls with Chronic Back Pain
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- XB
Xenofon Baraliakos, MD
Rheumazentrum Ruhrgebiet Herne
Herne, Germany
Abstract Poster Presenter(s)
Xenofon Baraliakos1, Imke Redeker2, Elena Bergmann3, Styliani Tsiami4 and Juergen Braun5, 1Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 2Rheumazentrum Ruhrgebiet Herne, Ruhr University Bochum, Bochum, Germany, 3Rheumazentrum Ruhrgebiet Herne, Ruhr-University Bochum, Herne, Germany, 4Rheumazentrum Ruhrgebiet, Herne, Ruhr-University Bochum, Herne, Germany, 5Rheumazentrum Ruhrgebiet, Herne, Germany
Background/Purpose: A fast response to NSAIDs is an important finding in the evaluation of clinical findings within the items comprising the ASAS classification criteria but also for the treatment decision for escalation to a bDMARD in patients with axial spondyloarthritis (axSpA). However, the differentiation of NSAID responses between patients with axSpA and degenerative or unspecific back pain is still unclear. We studied the differences in the velocity and magnitude of NSAID response velocity in patients with established bDMARD naïve axSpA vs. patients with other, non-inflammatory reasons of back pain.
Methods: Patients with axSpA without degenerative reasons for back pain or patients with degenerative or unspecific back pain presenting due to high levels of back pain (NRS≥4/10) were consecutively recruited. Assessments included clinical examination, laboratory tests and MRI of the lumbar spine. Previous NSAID intake was allowed only if it was taken in low doses without showing a clinical response, otherwise patients were NSAID naïve. Upon study inclusion, patients were treated with the maximum possible dose of an NSAID that they have reported to tolerate in lower doses in the past, independent of whether this was a Cox-2 inhibitor or a non-coxib. Assessment of response was performed using a standardized questionnaire after 2, 6, 12, 24, 36, 48 hours and after 1, 2 and 4 weeks. Any NSAID response was defined as improvement of pain >2/10 points and a good response to NSAIDs as an improvement >50% from the initial status.
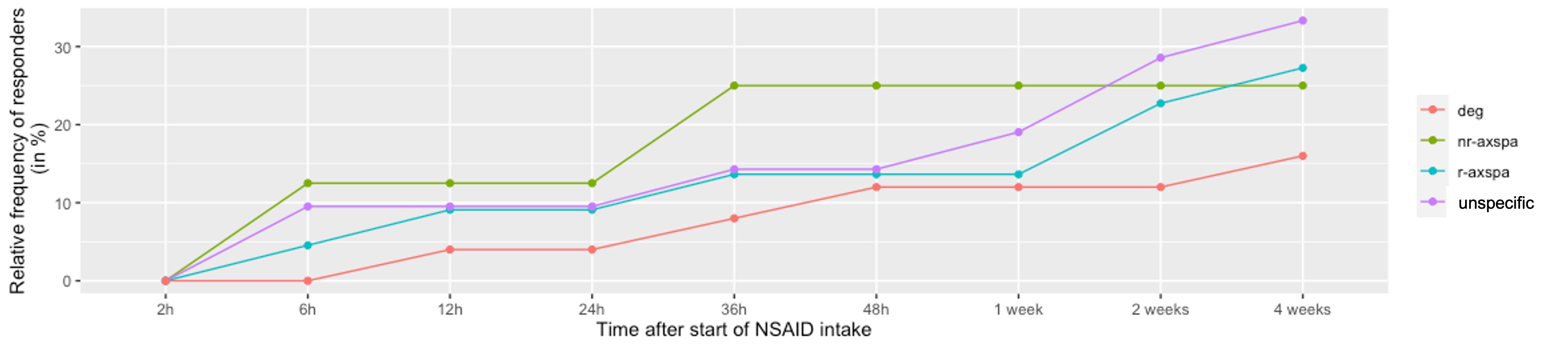
Results: A total of 68 patients with axSpA, 107 patients with degenerative back pain and 58 patients with unspecific back pain were included. The mean age was 42.7±10.7, 51.2±11.3, and 45.8±10.0 years, the main symptom duration 15.1±11.1, 16.1±12.6, and 11.9 ±10.1 years and the proportion of males was 57.4%, 19.6%, and 19.0% respectively. Inflammatory back pain was reported by 42 (75%), 48 (57.8%), and 29 (60.4%) patients, respectively and the mean pain score was 6.2±2.3, 6.7±1.8, and 6.2±1.8, respectively. In axSpA, the mean BASDAI and BASFI scores were 5.5±1.8 and 4.5±2.5, respectively. There was no difference in the cumulative response to NSAIDs between all three diagnoses, with an overall proportion of 27%-30% of patients showing improvement. However, better but not faster responses were found for the subgroups of patients with nr-axSpA (Fig. 1) and for the male patients in the entire axSpA group, while axSpA patients with systemic inflammatory activity defined by increased CRP showed lower rates of response as compared to non-inflammatory reasons of back pain diagnoses. All other subanalyses did not reveal any differences between axSpA patients and other non-inflammatory reasons of back pain.
Conclusion: In this prospective evaluation, the generally proposed better response of axSpA patients to treatment with high doses of NSAIDs as compared with non-inflammatory back pain was not confirmed, although the overall rate of responders was similar to previously reported rates. On the other hand, better responses were found in patients treated in the early (nr-axSpA) stage and in male patients. axSpA patients with increased CRP values showed lower rates of response.
Disclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; I. Redeker, None; E. Bergmann, None; S. Tsiami, None; J. Braun, None.
Background/Purpose: A fast response to NSAIDs is an important finding in the evaluation of clinical findings within the items comprising the ASAS classification criteria but also for the treatment decision for escalation to a bDMARD in patients with axial spondyloarthritis (axSpA). However, the differentiation of NSAID responses between patients with axSpA and degenerative or unspecific back pain is still unclear. We studied the differences in the velocity and magnitude of NSAID response velocity in patients with established bDMARD naïve axSpA vs. patients with other, non-inflammatory reasons of back pain.
Methods: Patients with axSpA without degenerative reasons for back pain or patients with degenerative or unspecific back pain presenting due to high levels of back pain (NRS≥4/10) were consecutively recruited. Assessments included clinical examination, laboratory tests and MRI of the lumbar spine. Previous NSAID intake was allowed only if it was taken in low doses without showing a clinical response, otherwise patients were NSAID naïve. Upon study inclusion, patients were treated with the maximum possible dose of an NSAID that they have reported to tolerate in lower doses in the past, independent of whether this was a Cox-2 inhibitor or a non-coxib. Assessment of response was performed using a standardized questionnaire after 2, 6, 12, 24, 36, 48 hours and after 1, 2 and 4 weeks. Any NSAID response was defined as improvement of pain >2/10 points and a good response to NSAIDs as an improvement >50% from the initial status.
Results: A total of 68 patients with axSpA, 107 patients with degenerative back pain and 58 patients with unspecific back pain were included. The mean age was 42.7±10.7, 51.2±11.3, and 45.8±10.0 years, the main symptom duration 15.1±11.1, 16.1±12.6, and 11.9 ±10.1 years and the proportion of males was 57.4%, 19.6%, and 19.0% respectively. Inflammatory back pain was reported by 42 (75%), 48 (57.8%), and 29 (60.4%) patients, respectively and the mean pain score was 6.2±2.3, 6.7±1.8, and 6.2±1.8, respectively. In axSpA, the mean BASDAI and BASFI scores were 5.5±1.8 and 4.5±2.5, respectively. There was no difference in the cumulative response to NSAIDs between all three diagnoses, with an overall proportion of 27%-30% of patients showing improvement. However, better but not faster responses were found for the subgroups of patients with nr-axSpA (Fig. 1) and for the male patients in the entire axSpA group, while axSpA patients with systemic inflammatory activity defined by increased CRP showed lower rates of response as compared to non-inflammatory reasons of back pain diagnoses. All other subanalyses did not reveal any differences between axSpA patients and other non-inflammatory reasons of back pain.
Conclusion: In this prospective evaluation, the generally proposed better response of axSpA patients to treatment with high doses of NSAIDs as compared with non-inflammatory back pain was not confirmed, although the overall rate of responders was similar to previously reported rates. On the other hand, better responses were found in patients treated in the early (nr-axSpA) stage and in male patients. axSpA patients with increased CRP values showed lower rates of response.

Disclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; I. Redeker, None; E. Bergmann, None; S. Tsiami, None; J. Braun, None.

