Back
Poster Session D
Immunobiology
Session: (1727–1749) T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
1747: Changes in the Number and Phenotype of Citrullinated-Antigen Specific T Cells Correlate with Treatment Outcome in Seropositive Rheumatoid Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EJ
Eddie James, PhD
Benaroya Research Institute at Virginia Mason
Seattle, WA, United States
Abstract Poster Presenter(s)
Cliff Rims1, Virginia Muir1, Anne Hocking1, Sylvia Posso1, Heather Bukiri2, Jeffrey Carlin3, Bernard Ng4, Peter Linsley1, Eddie James5 and Jane Buckner5, 1Benaroya Research Institute, Seattle, WA, 2University of California Los Angeles, Los Angeles, CA, 3Virginia Mason Medical Center, Seattle, WA, 4VA Puget Sound HCS, Seattle, WA, 5Benaroya Research Institute at Virginia Mason, Seattle, WA
Background/Purpose: In Rheumatoid arthritis (RA) citrullinated antigen reactive T cells are key drivers of disease, but knowledge about their relative number and phenotype remains limited. Therefore, we characterized antigen-specific T cells in peripheral blood samples from well-characterized subjects with RA.
Methods: We implemented an HLA class II tetramer panel to characterize epitope-specific T cells and applied a computational method (DISCOV-R) to define phenotypic clusters using cross-sectional samples from 66 seropositive RA subjects and 30 matched controls. We then applied these cluster definitions to longitudinal samples from seropositive RA subjects with active disease (initial RAPID3 > 2.3) stratified into responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response.
Results: Antigen specific T cells were detectable in seropositive RA at higher overall frequencies than in control subjects. In one subgroup, aggrecan, vimentin and fibrinogen specific T cells were predominant whereas α-enolase was predominant in a second sub-group. The global phenotypic landscape of CD4+ T cells included eight aligned clusters (AC), corresponding to known functional lineages. Among these, one Th17-like (CCR4+CCR6+) cluster was higher in RA subjects than controls and another Th17-like (CCR4midCCR6+) cluster increased with higher disease activity. T cell phenotype differed based on antigen specificity. Aggrecan and CILP-specific cells were enriched in individual Th2-like or naïve clusters, respectively, whereas alpha-enolase, vimentin and fibrinogen specific cells were more broadly distributed among several clusters. Generalized linear modeling of antigen specific T cell phenotypes revealed a significant enrichment of mature memory lineages with increased disease duration. Increased disease activity was positively correlated with Th1-like (CD45RA-CXCR3+) vimentin and fibrinogen specific T cells and negatively correlated with Tscm-like (CD45RA+CXCR3+) vimentin, fibrinogen, and CILP specific cells. Comparing responders and non-responders at baseline revealed differences in overall T cell number and the frequency of Th2-like (CD45RA-CCR4+) T cells. There were significant longitudinal changes in T cell frequency and phenotype. Non-responders exhibited increased overall numbers of antigen specific T cells over time, whereas numbers decreased in responders. Furthermore, a Tscm-like (CD45RA+CXCR3+) cluster increased over time in non-responders but exhibited no change in responders.
Conclusion: These findings show that the character of antigen specific T cells is influenced by epitope specificity, disease duration, and disease activity in RA. Furthermore, key baseline characteristics and longitudinal changes in the number and phenotype of antigen specific T cells correlate with treatment outcome. This supports the measurement of T cell phenotype to understand the effects of immunologic therapy and implicates potentially pathogenic populations of T cells that may accumulate in the absence of effective therapy.
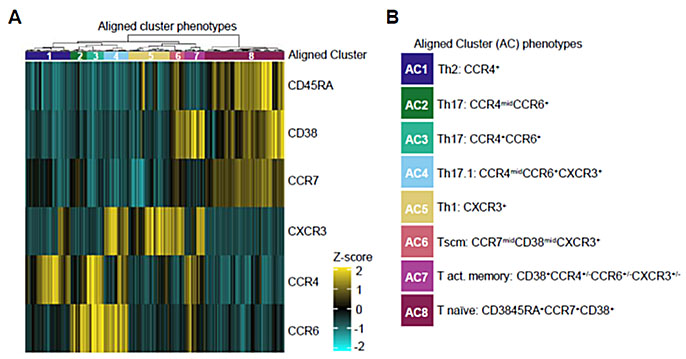 Figure 1. Aligned Clusters Define the CD4+ T cell Phenotypic Landscape. (A) Heat map showing surface marker expression across CD4+ T cell clusters from 66 RA subjects and 30 HC subjects, hierarchically clustered (Euclidean distance, Ward’s minimum variance linkage) with each of six phenotyping markers displayed as a z-score comparing mean cluster intensity to total CD4 T cell intensity for each subject. The resulting dendrogram is sliced into eight aligned clusters with color bar across top indicating aligned cluster assignment. (B) Suggested lineages of aligned clusters (AC) representing the CD4+ T cell landscape.
Figure 1. Aligned Clusters Define the CD4+ T cell Phenotypic Landscape. (A) Heat map showing surface marker expression across CD4+ T cell clusters from 66 RA subjects and 30 HC subjects, hierarchically clustered (Euclidean distance, Ward’s minimum variance linkage) with each of six phenotyping markers displayed as a z-score comparing mean cluster intensity to total CD4 T cell intensity for each subject. The resulting dendrogram is sliced into eight aligned clusters with color bar across top indicating aligned cluster assignment. (B) Suggested lineages of aligned clusters (AC) representing the CD4+ T cell landscape.
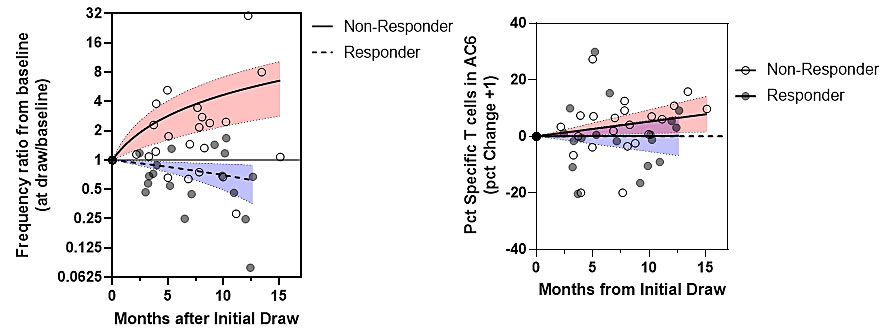 Figure 2. Antigen specific CD4 T cell phenotypes correlate with treatment response. The number and surface phenotype of antigen specific CD4+ T cells was examined through HLA tetramer and surface marker staining of blood samples from responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response. (A) The overall frequency of antigen-specific T cells significantly decreased in responders (p=0.0411, Spearman) but increased in non-responders (p=0.0165, Spearman). (B) The proportion of antigen specific T cells within Tscm-like (CD45RA+CXCR3+) cluster AC6 significantly increased in non-responders (p=0.0033, Spearman) but exhibited no change in responders (p=0.762, Spearman).
Figure 2. Antigen specific CD4 T cell phenotypes correlate with treatment response. The number and surface phenotype of antigen specific CD4+ T cells was examined through HLA tetramer and surface marker staining of blood samples from responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response. (A) The overall frequency of antigen-specific T cells significantly decreased in responders (p=0.0411, Spearman) but increased in non-responders (p=0.0165, Spearman). (B) The proportion of antigen specific T cells within Tscm-like (CD45RA+CXCR3+) cluster AC6 significantly increased in non-responders (p=0.0033, Spearman) but exhibited no change in responders (p=0.762, Spearman).
Disclosures: C. Rims, None; V. Muir, None; A. Hocking, None; S. Posso, None; H. Bukiri, None; J. Carlin, None; B. Ng, None; P. Linsley, None; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors.
Background/Purpose: In Rheumatoid arthritis (RA) citrullinated antigen reactive T cells are key drivers of disease, but knowledge about their relative number and phenotype remains limited. Therefore, we characterized antigen-specific T cells in peripheral blood samples from well-characterized subjects with RA.
Methods: We implemented an HLA class II tetramer panel to characterize epitope-specific T cells and applied a computational method (DISCOV-R) to define phenotypic clusters using cross-sectional samples from 66 seropositive RA subjects and 30 matched controls. We then applied these cluster definitions to longitudinal samples from seropositive RA subjects with active disease (initial RAPID3 > 2.3) stratified into responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response.
Results: Antigen specific T cells were detectable in seropositive RA at higher overall frequencies than in control subjects. In one subgroup, aggrecan, vimentin and fibrinogen specific T cells were predominant whereas α-enolase was predominant in a second sub-group. The global phenotypic landscape of CD4+ T cells included eight aligned clusters (AC), corresponding to known functional lineages. Among these, one Th17-like (CCR4+CCR6+) cluster was higher in RA subjects than controls and another Th17-like (CCR4midCCR6+) cluster increased with higher disease activity. T cell phenotype differed based on antigen specificity. Aggrecan and CILP-specific cells were enriched in individual Th2-like or naïve clusters, respectively, whereas alpha-enolase, vimentin and fibrinogen specific cells were more broadly distributed among several clusters. Generalized linear modeling of antigen specific T cell phenotypes revealed a significant enrichment of mature memory lineages with increased disease duration. Increased disease activity was positively correlated with Th1-like (CD45RA-CXCR3+) vimentin and fibrinogen specific T cells and negatively correlated with Tscm-like (CD45RA+CXCR3+) vimentin, fibrinogen, and CILP specific cells. Comparing responders and non-responders at baseline revealed differences in overall T cell number and the frequency of Th2-like (CD45RA-CCR4+) T cells. There were significant longitudinal changes in T cell frequency and phenotype. Non-responders exhibited increased overall numbers of antigen specific T cells over time, whereas numbers decreased in responders. Furthermore, a Tscm-like (CD45RA+CXCR3+) cluster increased over time in non-responders but exhibited no change in responders.
Conclusion: These findings show that the character of antigen specific T cells is influenced by epitope specificity, disease duration, and disease activity in RA. Furthermore, key baseline characteristics and longitudinal changes in the number and phenotype of antigen specific T cells correlate with treatment outcome. This supports the measurement of T cell phenotype to understand the effects of immunologic therapy and implicates potentially pathogenic populations of T cells that may accumulate in the absence of effective therapy.
 Figure 1. Aligned Clusters Define the CD4+ T cell Phenotypic Landscape. (A) Heat map showing surface marker expression across CD4+ T cell clusters from 66 RA subjects and 30 HC subjects, hierarchically clustered (Euclidean distance, Ward’s minimum variance linkage) with each of six phenotyping markers displayed as a z-score comparing mean cluster intensity to total CD4 T cell intensity for each subject. The resulting dendrogram is sliced into eight aligned clusters with color bar across top indicating aligned cluster assignment. (B) Suggested lineages of aligned clusters (AC) representing the CD4+ T cell landscape.
Figure 1. Aligned Clusters Define the CD4+ T cell Phenotypic Landscape. (A) Heat map showing surface marker expression across CD4+ T cell clusters from 66 RA subjects and 30 HC subjects, hierarchically clustered (Euclidean distance, Ward’s minimum variance linkage) with each of six phenotyping markers displayed as a z-score comparing mean cluster intensity to total CD4 T cell intensity for each subject. The resulting dendrogram is sliced into eight aligned clusters with color bar across top indicating aligned cluster assignment. (B) Suggested lineages of aligned clusters (AC) representing the CD4+ T cell landscape. Figure 2. Antigen specific CD4 T cell phenotypes correlate with treatment response. The number and surface phenotype of antigen specific CD4+ T cells was examined through HLA tetramer and surface marker staining of blood samples from responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response. (A) The overall frequency of antigen-specific T cells significantly decreased in responders (p=0.0411, Spearman) but increased in non-responders (p=0.0165, Spearman). (B) The proportion of antigen specific T cells within Tscm-like (CD45RA+CXCR3+) cluster AC6 significantly increased in non-responders (p=0.0033, Spearman) but exhibited no change in responders (p=0.762, Spearman).
Figure 2. Antigen specific CD4 T cell phenotypes correlate with treatment response. The number and surface phenotype of antigen specific CD4+ T cells was examined through HLA tetramer and surface marker staining of blood samples from responders (RAPID3 reduced to < 2.3 following change in therapy, n=9) and non-responders (maintained RAPID3 > 2.3, n=13) to identify T cell traits that correlate with treatment response. (A) The overall frequency of antigen-specific T cells significantly decreased in responders (p=0.0411, Spearman) but increased in non-responders (p=0.0165, Spearman). (B) The proportion of antigen specific T cells within Tscm-like (CD45RA+CXCR3+) cluster AC6 significantly increased in non-responders (p=0.0033, Spearman) but exhibited no change in responders (p=0.762, Spearman).Disclosures: C. Rims, None; V. Muir, None; A. Hocking, None; S. Posso, None; H. Bukiri, None; J. Carlin, None; B. Ng, None; P. Linsley, None; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors.

