Back
Poster Session D
Immunobiology
Session: (1727–1749) T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
1748: Deep Immune Profiling of Cytotoxic T Cells (CTL) from Patients with Ankylosing Spondylitis Revealed a Subset of CTL Co-Expressing PD-1 and TIGIT That Resists Immune Exhaustion
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MT
Michael Tang, PhD
University Health Network
Toronto, ON, Canada
Abstract Poster Presenter(s)
Michael Tang1, Addison Pacheco2, Zoya Qaiyum3, Melissa Lim1 and Robert Inman1, 1University Health Network, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada, 3Krembil Research Institute, Toronto, ON, Canada
Background/Purpose: Unresolved, chronic inflammation is a key feature of Ankylosing Spondylitis (AS) yet the immunological events perpetuating remain unclear. The strongest genetic association with AS is HLA-B27, a class I MHC allele, suggesting involvement of CTLs in AS pathogenesis. To date, the CTL compartment contributing to AS inflammation has not been fully defined. In the context of chronic inflammation, CTL exhaustion (often characterized by PD-1/TIGIT upregulation) represents a hyporesponsive state that limits excess effector function, which would otherwise cause inflammatory immunopathologies. Here, we sought to define how CTL dysregulation drives chronic inflammatory response in AS patients.
Methods: We performed extensive immunophenotyping of PBMCs and synovial fluid (SF) MCs, focusing on CTLs, using a 30+ parameter mass cytometry time-of-flight (CyTOF) panel. CTL in vitro functional assays were performed to assess cytotoxic functions.
Results: PD-1 and TIGIT expressions on peripheral CTLs are significantly lower (Mean= 17.2/25.5% of CD8 expressing PD-1/TIGIT respectively) in AS patients with active disease (BASDAI > 5.0) compared to healthy controls (Mean= 27.1/43.4%). Contrarily, PD-1 and TIGIT were highly upregulated on CTLs from SF of AS patients, suggesting evidence of strong immune activation and possible immune exhaustion. Unsupervised clustering analysis of CyTOF data identified two subsets of CTLs that co-express PD-1 and TIGIT based on CD127 expression. The CD127- subset (terminally exhausted) from SF expressed higher levels of GZMB, perforin, and CD38 compared to CD127+ PD-1+ CTLs (memory-like). Contrary to the CD127- CTL subset from PBMC, which produced substantially less IFNγ and TNFα upon stimulation, the CD127- CTL subset from SF retained the capacity to produce both cytokines.
Conclusion: We report a subset of CTLs in SF of AS patients co-expressing classical exhaustion markers (PD-1 & TIGIT) with downregulated CD127 expression. These dysregulated CTLs are highly cytotoxic and potentially exacerbate chronic inflammation in AS.
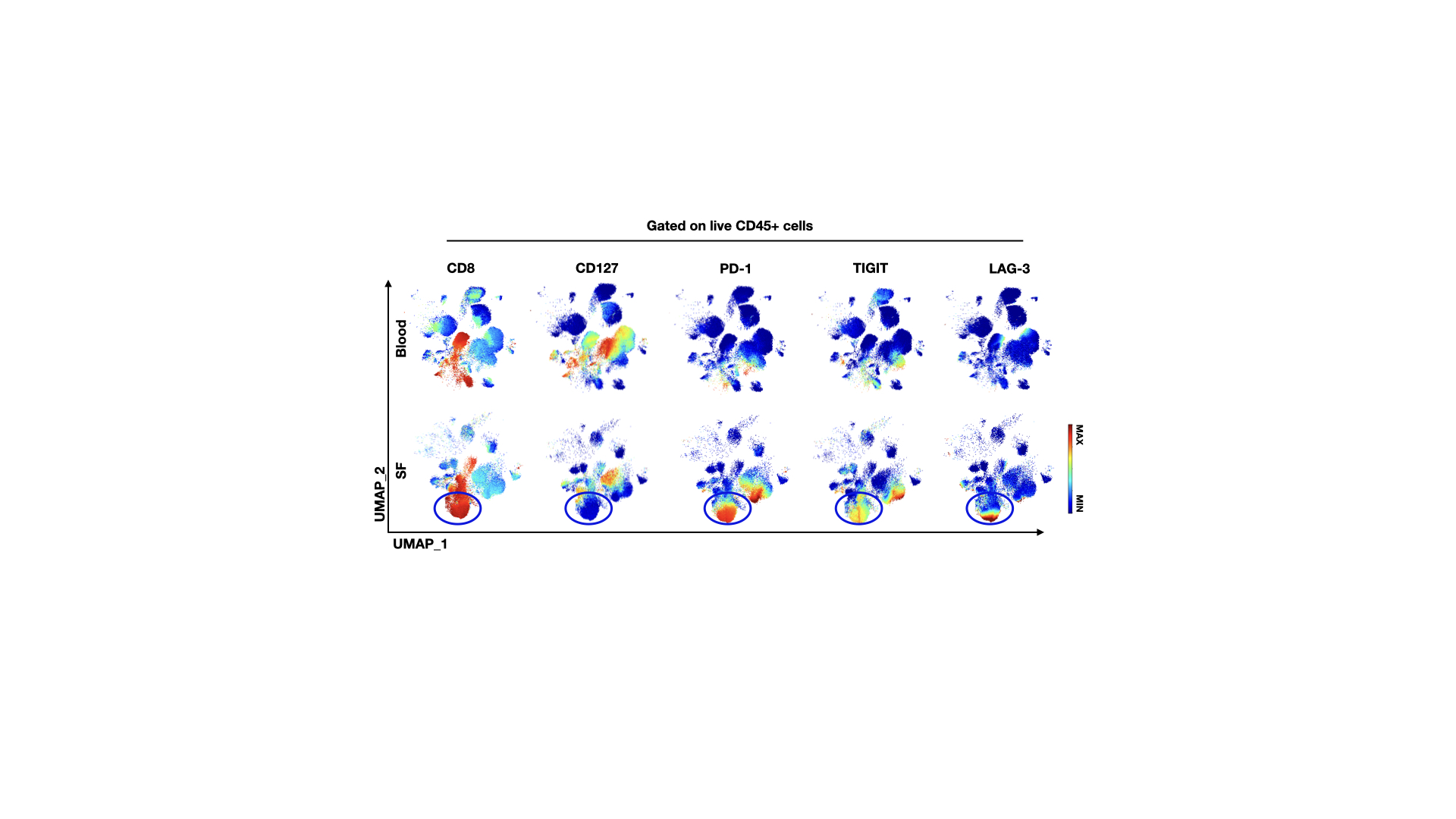 Live CD45+ cells from single file representations of paired AS SFMC and PBMC (n=8) were entered into UMAP algorithm for high-dimensional analysis of immune checkpoint molecules. Heat-maps display expression levels of indicated surface molecules.
Live CD45+ cells from single file representations of paired AS SFMC and PBMC (n=8) were entered into UMAP algorithm for high-dimensional analysis of immune checkpoint molecules. Heat-maps display expression levels of indicated surface molecules.
Disclosures: M. Tang, None; A. Pacheco, None; Z. Qaiyum, None; M. Lim, None; R. Inman, Novartis, Janssen, AbbVie/Abbott, Eli Lilly, Aria Pharmaceuticals, Amgen, Pfizer, Sandoz.
Background/Purpose: Unresolved, chronic inflammation is a key feature of Ankylosing Spondylitis (AS) yet the immunological events perpetuating remain unclear. The strongest genetic association with AS is HLA-B27, a class I MHC allele, suggesting involvement of CTLs in AS pathogenesis. To date, the CTL compartment contributing to AS inflammation has not been fully defined. In the context of chronic inflammation, CTL exhaustion (often characterized by PD-1/TIGIT upregulation) represents a hyporesponsive state that limits excess effector function, which would otherwise cause inflammatory immunopathologies. Here, we sought to define how CTL dysregulation drives chronic inflammatory response in AS patients.
Methods: We performed extensive immunophenotyping of PBMCs and synovial fluid (SF) MCs, focusing on CTLs, using a 30+ parameter mass cytometry time-of-flight (CyTOF) panel. CTL in vitro functional assays were performed to assess cytotoxic functions.
Results: PD-1 and TIGIT expressions on peripheral CTLs are significantly lower (Mean= 17.2/25.5% of CD8 expressing PD-1/TIGIT respectively) in AS patients with active disease (BASDAI > 5.0) compared to healthy controls (Mean= 27.1/43.4%). Contrarily, PD-1 and TIGIT were highly upregulated on CTLs from SF of AS patients, suggesting evidence of strong immune activation and possible immune exhaustion. Unsupervised clustering analysis of CyTOF data identified two subsets of CTLs that co-express PD-1 and TIGIT based on CD127 expression. The CD127- subset (terminally exhausted) from SF expressed higher levels of GZMB, perforin, and CD38 compared to CD127+ PD-1+ CTLs (memory-like). Contrary to the CD127- CTL subset from PBMC, which produced substantially less IFNγ and TNFα upon stimulation, the CD127- CTL subset from SF retained the capacity to produce both cytokines.
Conclusion: We report a subset of CTLs in SF of AS patients co-expressing classical exhaustion markers (PD-1 & TIGIT) with downregulated CD127 expression. These dysregulated CTLs are highly cytotoxic and potentially exacerbate chronic inflammation in AS.
 Live CD45+ cells from single file representations of paired AS SFMC and PBMC (n=8) were entered into UMAP algorithm for high-dimensional analysis of immune checkpoint molecules. Heat-maps display expression levels of indicated surface molecules.
Live CD45+ cells from single file representations of paired AS SFMC and PBMC (n=8) were entered into UMAP algorithm for high-dimensional analysis of immune checkpoint molecules. Heat-maps display expression levels of indicated surface molecules.Disclosures: M. Tang, None; A. Pacheco, None; Z. Qaiyum, None; M. Lim, None; R. Inman, Novartis, Janssen, AbbVie/Abbott, Eli Lilly, Aria Pharmaceuticals, Amgen, Pfizer, Sandoz.

