Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1916: Defining Early-stage Knee Osteoarthritis: A Scoping Review
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jean Liew, MD, MS
Fellow Physician
Boston University
University of Washington
Boston, MA, United States
Abstract Poster Presenter(s)
Jean Liew1, Lauren King2, Armaghan Mahmoudian3, Qiuke Wang4, Tuhina Neogi1 and Gillian Hawker2, 1Boston University School of Medicine, Boston, MA, 2University of Toronto, Toronto, ON, Canada, 3Lund University, Lund, Sweden, 4Erasmus Medical Center, Rotterdam, Netherlands
Background/Purpose: Clinical trials of disease-modifying agents in knee osteoarthritis (OA) have largely failed to date. Identifying individuals with knee OA at an earlier stage in the disease process may provide a window of opportunity in which interventions may prove fruitful. However, there is no consensus definition for early-stage knee OA to enable trial enrollment. To better define the construct of early-stage knee OA (EsKOA), we sought to identify how early knee OA has been defined in the literature to date, and the most frequently used elements within the definitions.
Methods: We conducted a scoping literature review using PubMed from database inception to March 18, 2022. We included human studies in which early-stage knee OA was included as a study population or outcome of interest, without language exclusions. Two independent reviewers screened each abstract and full text article. Data were extracted from full text articles and included study characteristics, the EsKOA definition, and the inclusion of demographic, symptoms, standardized patient-reported measures, physical examination, laboratory, imaging, performance-based measures, and gross inspection/histopathologic domains as well as individual elements within the EsKOA definition.
Results: We identified 2289 articles in PubMed, of which 210 were included for data abstraction. Of these, 58 consisted of duplicate cohorts with duplicate definitions of EsKOA and were excluded. Of the remaining 152 articles (32 interventional, 89 observational, 3 qualitative, 24 translational/basic science, 3 solely for criteria development), most were performed in Asia (n=54) or Europe (n=48). All were published after 1990. An EsKOA definition was used as inclusion criterion in 142 studies, for outcome definition in 5, and for development of new criteria in 5.
A detailed definition of EsKOA was lacking in 63 studies. EsKOA was defined by Kellgren-Lawrence (KL) radiographic OA grade alone in 39 (with many including KL grade 2), or by previously developed early OA criteria by Luyten, et al. in 13 studies. Fulfilling the 1986 ACR classification criteria for OA was included in the definition in 19 studies.
Demographic domains were included in the EsKOA definition in 30 studies, with 28 including an age cutoff. Symptomatic domains were included in 73 studies and the most common elements were presence of knee pain (49) and pain duration (25). Exam domains were included in 23 studies, with crepitus (14), joint tenderness (12), bony enlargement (6) as the most common elements. Imaging was included in 130 studies (most common element KL grade), gross inspection/histopathologic in 20 (most common element cartilage abnormalities). Only 8 studies included laboratory values, 13 other patient-reported outcomes, 1 performance-based measures.
Conclusion: Although EsKOA has been studied in the published literature, it remains variably defined. Many definitions included fulfilling 1986 ACR classification criteria or KL grades 2+, which reflects established OA, suggesting a failure to truly capture EsKOA. These findings support the need to develop evidence-supported, validated classification criteria for EsKOA.
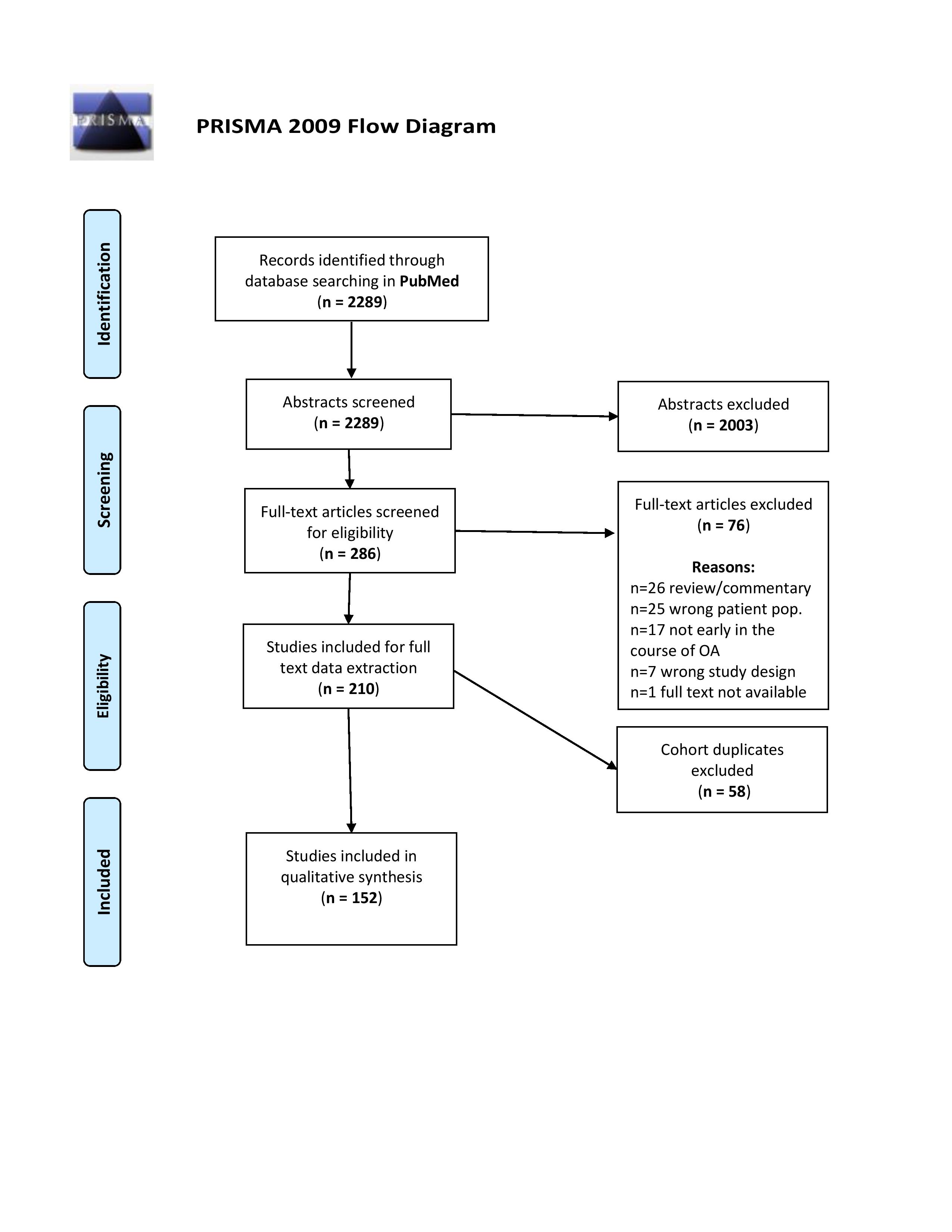 PRISMA flow diagram of study inclusion.
PRISMA flow diagram of study inclusion.
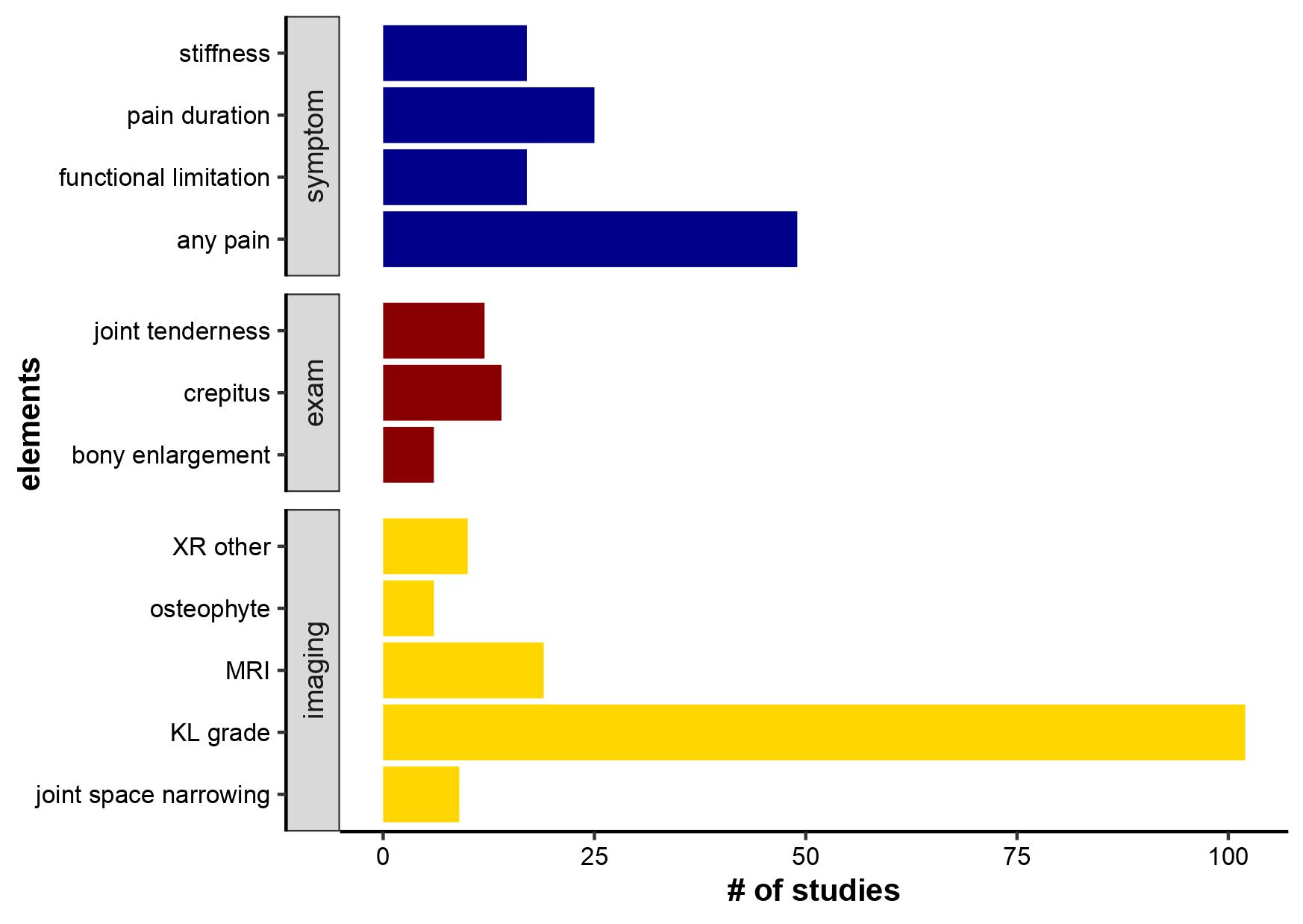 Symptom, exam, and imaging elements included in EsKOA definitions in the studies identified by our scoping review
Symptom, exam, and imaging elements included in EsKOA definitions in the studies identified by our scoping review
Disclosures: J. Liew, None; L. King, None; A. Mahmoudian, None; Q. Wang, None; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; G. Hawker, None.
Background/Purpose: Clinical trials of disease-modifying agents in knee osteoarthritis (OA) have largely failed to date. Identifying individuals with knee OA at an earlier stage in the disease process may provide a window of opportunity in which interventions may prove fruitful. However, there is no consensus definition for early-stage knee OA to enable trial enrollment. To better define the construct of early-stage knee OA (EsKOA), we sought to identify how early knee OA has been defined in the literature to date, and the most frequently used elements within the definitions.
Methods: We conducted a scoping literature review using PubMed from database inception to March 18, 2022. We included human studies in which early-stage knee OA was included as a study population or outcome of interest, without language exclusions. Two independent reviewers screened each abstract and full text article. Data were extracted from full text articles and included study characteristics, the EsKOA definition, and the inclusion of demographic, symptoms, standardized patient-reported measures, physical examination, laboratory, imaging, performance-based measures, and gross inspection/histopathologic domains as well as individual elements within the EsKOA definition.
Results: We identified 2289 articles in PubMed, of which 210 were included for data abstraction. Of these, 58 consisted of duplicate cohorts with duplicate definitions of EsKOA and were excluded. Of the remaining 152 articles (32 interventional, 89 observational, 3 qualitative, 24 translational/basic science, 3 solely for criteria development), most were performed in Asia (n=54) or Europe (n=48). All were published after 1990. An EsKOA definition was used as inclusion criterion in 142 studies, for outcome definition in 5, and for development of new criteria in 5.
A detailed definition of EsKOA was lacking in 63 studies. EsKOA was defined by Kellgren-Lawrence (KL) radiographic OA grade alone in 39 (with many including KL grade 2), or by previously developed early OA criteria by Luyten, et al. in 13 studies. Fulfilling the 1986 ACR classification criteria for OA was included in the definition in 19 studies.
Demographic domains were included in the EsKOA definition in 30 studies, with 28 including an age cutoff. Symptomatic domains were included in 73 studies and the most common elements were presence of knee pain (49) and pain duration (25). Exam domains were included in 23 studies, with crepitus (14), joint tenderness (12), bony enlargement (6) as the most common elements. Imaging was included in 130 studies (most common element KL grade), gross inspection/histopathologic in 20 (most common element cartilage abnormalities). Only 8 studies included laboratory values, 13 other patient-reported outcomes, 1 performance-based measures.
Conclusion: Although EsKOA has been studied in the published literature, it remains variably defined. Many definitions included fulfilling 1986 ACR classification criteria or KL grades 2+, which reflects established OA, suggesting a failure to truly capture EsKOA. These findings support the need to develop evidence-supported, validated classification criteria for EsKOA.
 PRISMA flow diagram of study inclusion.
PRISMA flow diagram of study inclusion.  Symptom, exam, and imaging elements included in EsKOA definitions in the studies identified by our scoping review
Symptom, exam, and imaging elements included in EsKOA definitions in the studies identified by our scoping reviewDisclosures: J. Liew, None; L. King, None; A. Mahmoudian, None; Q. Wang, None; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; G. Hawker, None.

