Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1763: Different Humoral but Similar Cellular Responses of Patients with Autoimmune Inflammatory Rheumatic Diseases Under Disease-modifying Anti-rheumatic Drugs After COVID-19 Vaccination
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Ioana Andreica, MD
Rheumazentrum Ruhrgebiet Herne
Herne, Germany
Abstract Poster Presenter(s)
Ioana Andreica1, Arturo Blazquez-Navarro2, Jan Sokolar3, Moritz Anft4, Uta Kiltz5, Stephanie Pfaender6, Elena Vidal Blanco7, Timm Westhoff8, Nina Babel8, Ulrik Stervbo9 and Xenofon Baraliakos10, 1Rheumazentrum Ruhrgebiet, Ruhr-Universität-Bochum, Herne, Germany, 2Charité Universitätsmedizin Berlin, Berlin Center for Advanced Therapies, Berlin, Germany, 3Rheumazentrum Ruhrgebiet Herne, Ruhr Universität Bochum, Herne, Germany, 4Marienhospital Herne - Klinik Mitte Medizinische Klinik I - Gastroenterologie, Nieren- und Hochdruckkrankheiten, Center for Translational Medicine and Immune Diagnostics Laboratory, Medical Department I, Herne and Ruhr-Universit t Bochum, Medical Department I, Bochum, Germany, 5Rheumazentrum Ruhrgebiet, Herne, Germany, 6Ruhr-Universität Bochum, Medical Department I, Bochum, Germany, 7Ruhr-University Bochum, Department of Molecular and Medical Virology, Bochum, Germany, 8Marienhospital Herne, Ruhr-University Bochum, Herne, Germany, 9Marienhospital Herne - Klinik Mitte Medizinische Klinik I - Gastroenterologie, Nieren- und Hochdruckkrankheiten, Center for Translational Medicine and Immune Diagnostics Laboratory, Medical Department I, Herne and Ruhr-Universität Bochum, Medical Department I, Bochum, Germany, 10Rheumazentrum Ruhrgebiet Herne, Herne, Germany
Background/Purpose: The interplay between humoral and cellular response after vaccination against SARS-CoV-2 in patients (pts.) with autoimmune inflammatory rheumatic diseases (AIRD) remains unknown. To investigate the impact of different immunosuppressive therapies on the development of humoral and cellular immune responses to full 2-dose SARS-CoV-2 vaccination in AIRD pts. with stable low disease activity.
Methods: The immune reactivity to COVID-19 vaccination was investigated in a prospectively recruited AIRD cohort with rheumatoid arthritis, axial spondyloarthritis or psoriatic arthritis which received a therapy with IL-17i, TNFi, JAKi or MTX (alone or in combination). Almost all patients received mRNA-based vaccine, only 4 patients had a heterologous scheme. Anti-spike(S) antibodies(ab.) and sera neutralizing capacity (neutralization dilution 50; ND50) were measured 4 weeks after the first (prime+4w) and 4 weeks after the second vaccination (boost+4w). Vaccine-specific cellular immunity was evaluated by quantifying expression of activation markers on T cells as well as their production of key cytokines, at prime+4w and boost+4w.
Results: Overall, a total of 92 pts. were included in the final cohort. 31 (33.7%) pts. were on TNFi, 24 (26.1%) on IL-17i, 24 (26.1%) on JAKi, each group encompassing pts. receiving drug inhibitors alone or in combination with MTX.13 (14.1%) were treated with MTX alone. The median time between the vaccination and blood sampling was 31 [IQR: 28-34] days after prime+4w and 28 [IRQ: 28-28] days after boost+4w. Although at prime+4w only 34/90 (37.8%) of pts. presented neutralizing ab., the majority (86/91, 94.5%), developed them at boost+4w. The highest neutralization titer developed the pts. on IL-17i both at prime+4w (74 [IQR: 13-91]) and boost+4w (798 [IQR: 511- 1344]), while no statistically significant differences were found in the neutralization titer at boost+4w for the TNFi, JAKi, and MTX groups: 207 ND50 [IQR: 120-576], 319 [IQR: 133-461] and 749 [IQR: 264-1920], respectively. 81/90 (90.0%) pts. developed IgG ab. against SARS-CoV-2 S-protein at prime+4w and 91/92 (98.9%) at boost+4w. Pts. receiving IL-17i developed higher ab. titers (8295 U/mL [IQR: 4586-11,237]) compared to the other three groups: JAKi (4405 U/mL [IQR: 1436-7265], TNFi (2313 [IQR: 1156-3630] U/mL) and MTX (2010 U/mL [IQR: 693-9254]). Neutralization capacity correlated well with the titer of anti-S ab. at both timepoints. Coadministration of biologic/tsDMARDs and MTX led to lower titers compared to biologic/tsDMARDs monotherapy. All therapies left frequencies of CD154+CD137+ CD4+ T cells and CD137+ CD8+ T cells at prime+4w and boost+4w unchanged. Polyfunctionality and T cell cytokine profiles across therapies did not significantly vary at boost+4w.
Conclusion: Even after insufficient seroconversion for neutralizing capacity and ab. response against SARS-CoV-2 S-proteins between pts. of different mod of action agents, particularly for MTX and JAKi after first vaccination, a second vaccination covered almost all pts. regardless of DMARDs therapy, with better outcomes in those on IL-17i. T cell immunity revealed similar frequencies of activated T cells in all modes of action after the second vaccination.
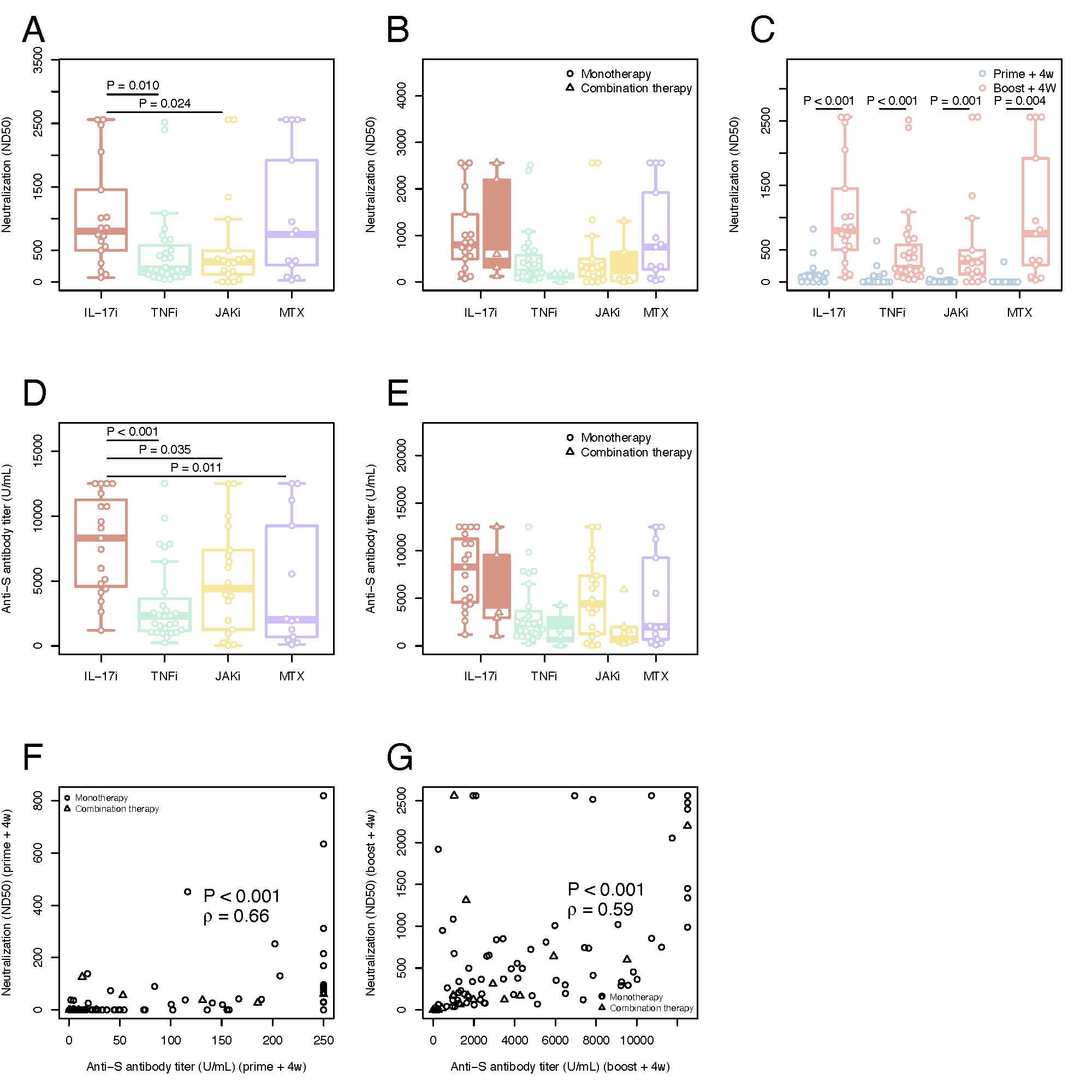 Figure 1: Serological immune responses and neutralization titers against SARS-CoV-2 wild variant in time and by different therapies
Figure 1: Serological immune responses and neutralization titers against SARS-CoV-2 wild variant in time and by different therapies
Neutralization titers (ND50) against SARS-CoV-2 in plasma A) SARS-CoV-2 neutralization antibodies at boost+4w for all four groups of patients on monotherapy B) Comparison between SARS-CoV-2 neutralization antibodies at boost+4w for all four groups of patients on monotherapy (unfilled plots) and combination therapies (filled colored plots) C) Kinetic of SARS-CoV-2 neutralization antibodies for all four groups of patients on monotherapy with IL17i, TNFi, JAKi and MTX. Serological immune responses against SARS-CoV-2 D) Spike-specific IgG titers at boost+4w for all four groups of patients on monotherapy E) Comparison between spike-specific IgG titers at boost+4w for all four groups of patients on monotherapy (unfilled plots) and combination therapies (filled colored plots) F) Correlation of spike-specific IgG titers and SARS-CoV-2 neutralization antibodies at prime+4w G) Correlation of spike-specific IgG titers and SARS-CoV-2 neutralization antibodies at boost+4w. The box plots indicate the 75th, 50th, and 25th quantile, and the whiskers have a maximum length of 1.5 times the interquartile range. Each point represents individual values, small triangles represent the additional patients on combination therapy. The following number of patients are presented: IL17i (n=19 prime+4w and n=18 boost+4w), TNFi (n=27 prime+4w, n=27 boost+4w), JAKi (n=18 prime+4w, n=18 boost+4w) and MTX (n=11 prime+4w, n=13 boost+4w), IL17i/MTX (n=5 boost+4w), TNFi/MTX (n=4 boost+4w), JAKi/MTX (n=6 boost+4w). ND50=50% inhibitory dilution. U/ml=units/mililiter. IL=interleukin. TNF=tumor necrosis factor. JAK= Janus kinase inhibitor. MTX=methotrexate. ρ=Spearman’s correlation coefficient.
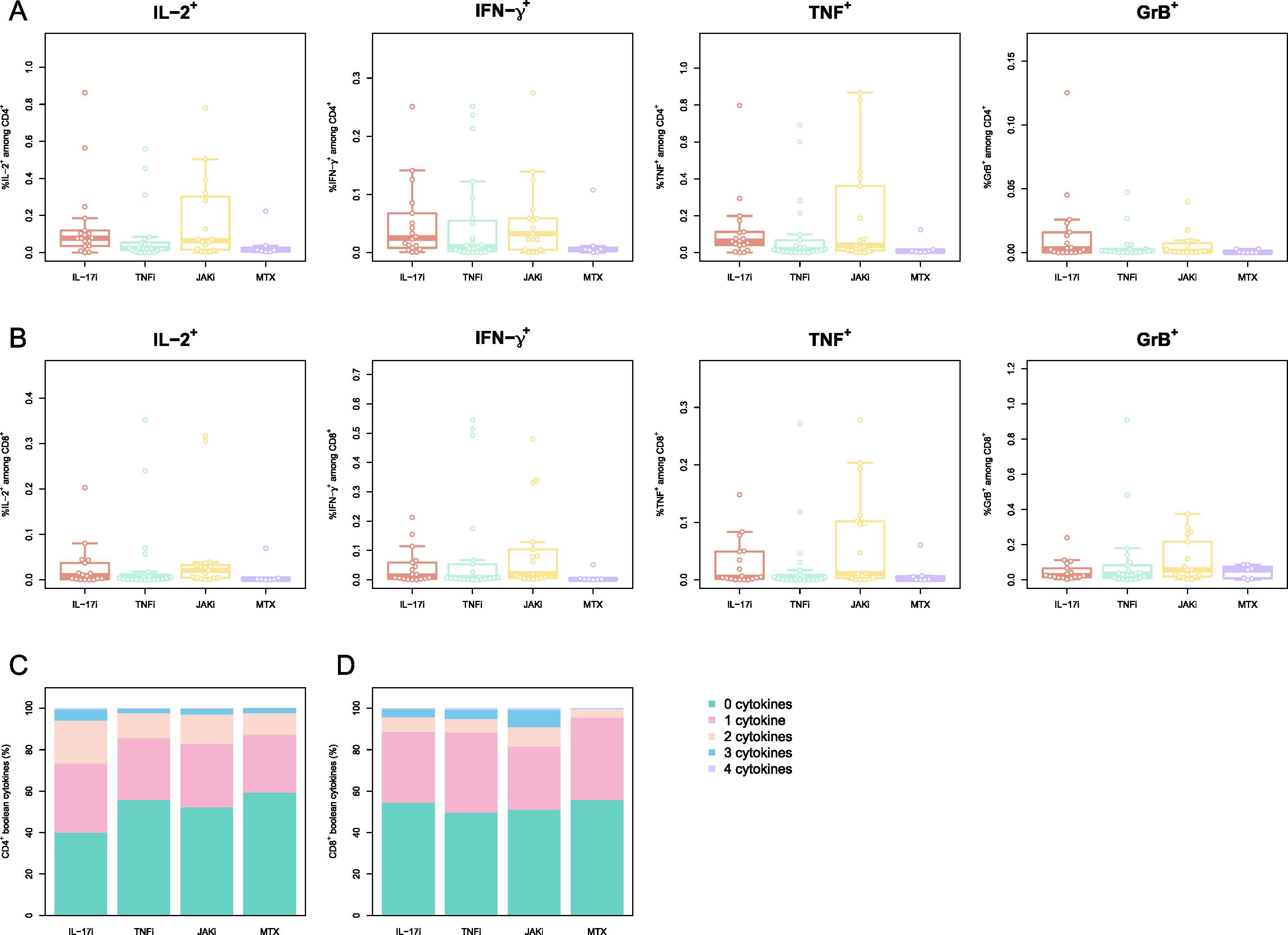 Figure 2 Memory phenotype of activated cells is perturbed by different therapies:
Figure 2 Memory phenotype of activated cells is perturbed by different therapies:
PBMCs were stimulated with peptides spanning the SARS-CoV-2 Spike-protein and activated cells were evaluated by multiparametric flow cytometry. Phenotype of CD4+ T cells reactive to SARS-CoV-2 S-protein A) Memory/Naive phenotype of SARS-CoV-2 Spike-reactive CD4+ T cells at boost+4w. Naïve (CD45RA+CCR7+), central memory (CM; CD45RA–CCR7+), effector memory (EM; CD45RA–CCR7–), and EM expressing CD45RA (TEMRA; CD45RA+CCR7–). Phenotype of CD8+ T cells reactive to SARS-CoV-2 S-protein B) Memory/Naive phenotype of SARS-CoV-2 Spike-reactive CD8+ T cells at boost+4w. The cell populations are defined as for A) C-D) Frequency of the cytokines IL-2, IFN-γ, TNF and GrB for SARS-CoV-2 Spike-reactive CD4+ (C) CD8+ (D) T cells at boost+4w. E-F) Boolean combinations of assayed cytokines for CD4+ and CD8+ T cells. The box plots indicate the 75th, 50th, and 25th quantile, and the whiskers have a maximum length of 1.5 times the interquartile range. Each point represents a patient. IL=interleukin. TNF=tumor necrosis factor. JAK= Janus kinase inhibitor. MTX=methotrexate. Gr= granzyme
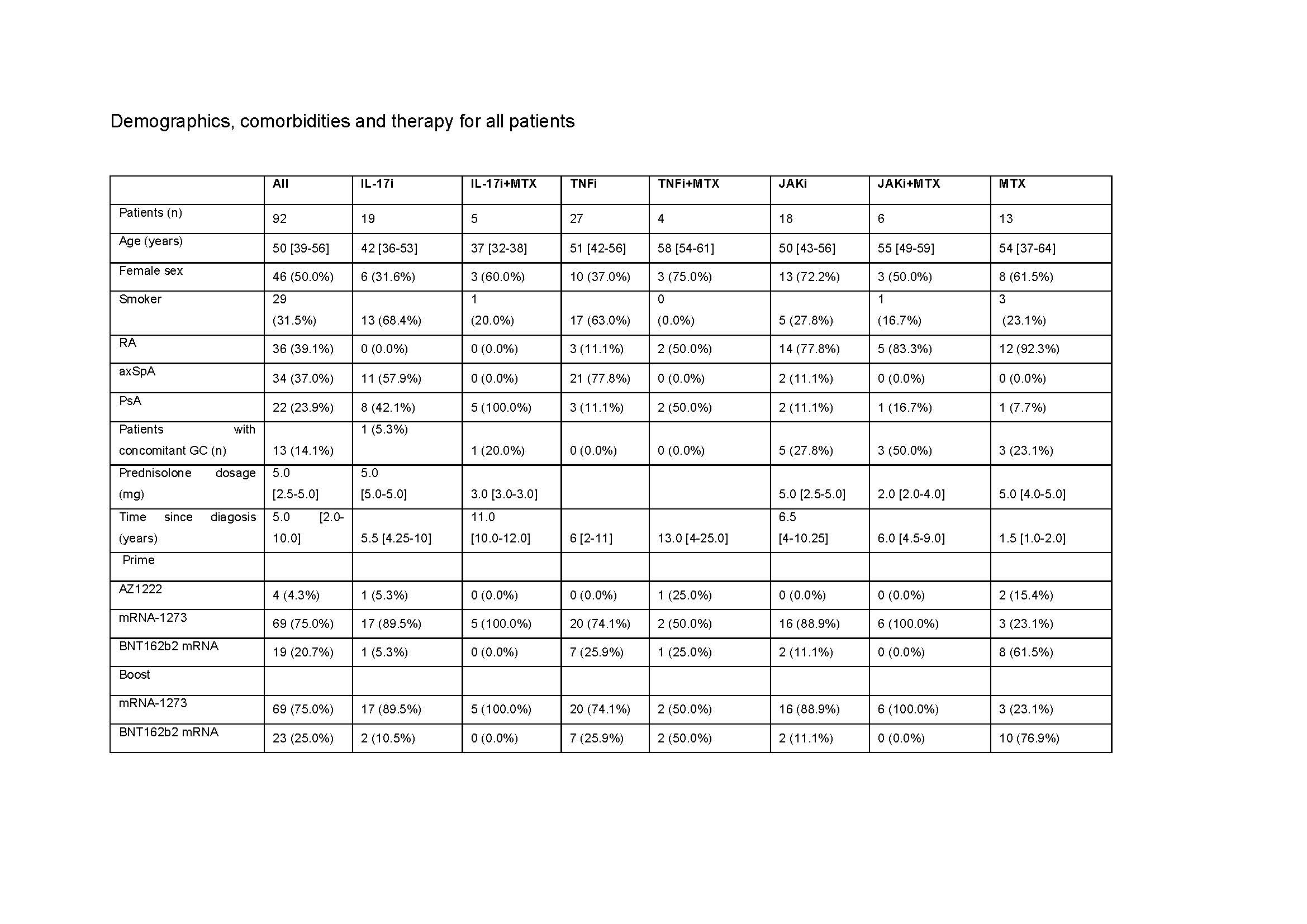 Table 1. Demographics, comorbidities and therapy for all patients
Table 1. Demographics, comorbidities and therapy for all patients
RA: rheumatoid arthritis, axSpA: axial spondyloathritis, PsA: psoriatic arthritis, GC: glucocorticoid, mg: milligram, IL: interleukin, i: inhibitor, MTX: methotrexate: TNF: tumor necrosis factor, JAK: janus kinase. For quantitative variables, data are provided as median [IQR], while for categorical variables the count (% frequency) is provided
Disclosures: I. Andreica, None; A. Blazquez-Navarro, None; J. Sokolar, None; M. Anft, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; S. Pfaender, None; E. Vidal Blanco, None; T. Westhoff, None; N. Babel, None; U. Stervbo, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi.
Background/Purpose: The interplay between humoral and cellular response after vaccination against SARS-CoV-2 in patients (pts.) with autoimmune inflammatory rheumatic diseases (AIRD) remains unknown. To investigate the impact of different immunosuppressive therapies on the development of humoral and cellular immune responses to full 2-dose SARS-CoV-2 vaccination in AIRD pts. with stable low disease activity.
Methods: The immune reactivity to COVID-19 vaccination was investigated in a prospectively recruited AIRD cohort with rheumatoid arthritis, axial spondyloarthritis or psoriatic arthritis which received a therapy with IL-17i, TNFi, JAKi or MTX (alone or in combination). Almost all patients received mRNA-based vaccine, only 4 patients had a heterologous scheme. Anti-spike(S) antibodies(ab.) and sera neutralizing capacity (neutralization dilution 50; ND50) were measured 4 weeks after the first (prime+4w) and 4 weeks after the second vaccination (boost+4w). Vaccine-specific cellular immunity was evaluated by quantifying expression of activation markers on T cells as well as their production of key cytokines, at prime+4w and boost+4w.
Results: Overall, a total of 92 pts. were included in the final cohort. 31 (33.7%) pts. were on TNFi, 24 (26.1%) on IL-17i, 24 (26.1%) on JAKi, each group encompassing pts. receiving drug inhibitors alone or in combination with MTX.13 (14.1%) were treated with MTX alone. The median time between the vaccination and blood sampling was 31 [IQR: 28-34] days after prime+4w and 28 [IRQ: 28-28] days after boost+4w. Although at prime+4w only 34/90 (37.8%) of pts. presented neutralizing ab., the majority (86/91, 94.5%), developed them at boost+4w. The highest neutralization titer developed the pts. on IL-17i both at prime+4w (74 [IQR: 13-91]) and boost+4w (798 [IQR: 511- 1344]), while no statistically significant differences were found in the neutralization titer at boost+4w for the TNFi, JAKi, and MTX groups: 207 ND50 [IQR: 120-576], 319 [IQR: 133-461] and 749 [IQR: 264-1920], respectively. 81/90 (90.0%) pts. developed IgG ab. against SARS-CoV-2 S-protein at prime+4w and 91/92 (98.9%) at boost+4w. Pts. receiving IL-17i developed higher ab. titers (8295 U/mL [IQR: 4586-11,237]) compared to the other three groups: JAKi (4405 U/mL [IQR: 1436-7265], TNFi (2313 [IQR: 1156-3630] U/mL) and MTX (2010 U/mL [IQR: 693-9254]). Neutralization capacity correlated well with the titer of anti-S ab. at both timepoints. Coadministration of biologic/tsDMARDs and MTX led to lower titers compared to biologic/tsDMARDs monotherapy. All therapies left frequencies of CD154+CD137+ CD4+ T cells and CD137+ CD8+ T cells at prime+4w and boost+4w unchanged. Polyfunctionality and T cell cytokine profiles across therapies did not significantly vary at boost+4w.
Conclusion: Even after insufficient seroconversion for neutralizing capacity and ab. response against SARS-CoV-2 S-proteins between pts. of different mod of action agents, particularly for MTX and JAKi after first vaccination, a second vaccination covered almost all pts. regardless of DMARDs therapy, with better outcomes in those on IL-17i. T cell immunity revealed similar frequencies of activated T cells in all modes of action after the second vaccination.
 Figure 1: Serological immune responses and neutralization titers against SARS-CoV-2 wild variant in time and by different therapies
Figure 1: Serological immune responses and neutralization titers against SARS-CoV-2 wild variant in time and by different therapiesNeutralization titers (ND50) against SARS-CoV-2 in plasma A) SARS-CoV-2 neutralization antibodies at boost+4w for all four groups of patients on monotherapy B) Comparison between SARS-CoV-2 neutralization antibodies at boost+4w for all four groups of patients on monotherapy (unfilled plots) and combination therapies (filled colored plots) C) Kinetic of SARS-CoV-2 neutralization antibodies for all four groups of patients on monotherapy with IL17i, TNFi, JAKi and MTX. Serological immune responses against SARS-CoV-2 D) Spike-specific IgG titers at boost+4w for all four groups of patients on monotherapy E) Comparison between spike-specific IgG titers at boost+4w for all four groups of patients on monotherapy (unfilled plots) and combination therapies (filled colored plots) F) Correlation of spike-specific IgG titers and SARS-CoV-2 neutralization antibodies at prime+4w G) Correlation of spike-specific IgG titers and SARS-CoV-2 neutralization antibodies at boost+4w. The box plots indicate the 75th, 50th, and 25th quantile, and the whiskers have a maximum length of 1.5 times the interquartile range. Each point represents individual values, small triangles represent the additional patients on combination therapy. The following number of patients are presented: IL17i (n=19 prime+4w and n=18 boost+4w), TNFi (n=27 prime+4w, n=27 boost+4w), JAKi (n=18 prime+4w, n=18 boost+4w) and MTX (n=11 prime+4w, n=13 boost+4w), IL17i/MTX (n=5 boost+4w), TNFi/MTX (n=4 boost+4w), JAKi/MTX (n=6 boost+4w). ND50=50% inhibitory dilution. U/ml=units/mililiter. IL=interleukin. TNF=tumor necrosis factor. JAK= Janus kinase inhibitor. MTX=methotrexate. ρ=Spearman’s correlation coefficient.
 Figure 2 Memory phenotype of activated cells is perturbed by different therapies:
Figure 2 Memory phenotype of activated cells is perturbed by different therapies:PBMCs were stimulated with peptides spanning the SARS-CoV-2 Spike-protein and activated cells were evaluated by multiparametric flow cytometry. Phenotype of CD4+ T cells reactive to SARS-CoV-2 S-protein A) Memory/Naive phenotype of SARS-CoV-2 Spike-reactive CD4+ T cells at boost+4w. Naïve (CD45RA+CCR7+), central memory (CM; CD45RA–CCR7+), effector memory (EM; CD45RA–CCR7–), and EM expressing CD45RA (TEMRA; CD45RA+CCR7–). Phenotype of CD8+ T cells reactive to SARS-CoV-2 S-protein B) Memory/Naive phenotype of SARS-CoV-2 Spike-reactive CD8+ T cells at boost+4w. The cell populations are defined as for A) C-D) Frequency of the cytokines IL-2, IFN-γ, TNF and GrB for SARS-CoV-2 Spike-reactive CD4+ (C) CD8+ (D) T cells at boost+4w. E-F) Boolean combinations of assayed cytokines for CD4+ and CD8+ T cells. The box plots indicate the 75th, 50th, and 25th quantile, and the whiskers have a maximum length of 1.5 times the interquartile range. Each point represents a patient. IL=interleukin. TNF=tumor necrosis factor. JAK= Janus kinase inhibitor. MTX=methotrexate. Gr= granzyme
 Table 1. Demographics, comorbidities and therapy for all patients
Table 1. Demographics, comorbidities and therapy for all patientsRA: rheumatoid arthritis, axSpA: axial spondyloathritis, PsA: psoriatic arthritis, GC: glucocorticoid, mg: milligram, IL: interleukin, i: inhibitor, MTX: methotrexate: TNF: tumor necrosis factor, JAK: janus kinase. For quantitative variables, data are provided as median [IQR], while for categorical variables the count (% frequency) is provided
Disclosures: I. Andreica, None; A. Blazquez-Navarro, None; J. Sokolar, None; M. Anft, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; S. Pfaender, None; E. Vidal Blanco, None; T. Westhoff, None; N. Babel, None; U. Stervbo, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi.

