Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Etiology and Pathogenesis (0498–0501)
0498: A Longitudinal Assessment of Citrulline-specific CD4 T Cells in CCP+ at Risk Subjects and Patients with New-onset Rheumatoid Arthritis
Saturday, November 12, 2022
3:00 PM – 3:10 PM Eastern Time
Location: Room 204
- JS
JING SONG, MD, PhD
Benaroya Research Institute
Seattle, WA, United States
Presenting Author(s)
Jing Song1, Scott Presnell1, Cliff Rims2, Matthew Dufort1, Hannah DeBerg1, Peter Linsley2, V. Michael Holers3, Kevin D Deane4, Eddie James1 and Jane Buckner1, 1Benaroya Research Institute at Virginia Mason, Seattle, WA, 2Benaroya Research Institute, Seattle, WA, 3University of Colorado, Denver, CO, 4University of Colorado Denver Anschutz Medical Campus, Denver, CO
Background/Purpose: Rheumatoid arthritis (RA) is an autoimmune disease, in which autoantibodies targeting citrullinated (cit) peptides can be detected prior to diagnosis. We previously reported an increase in cit-specific CD4+ T cells in HLA-DRB1*0401 CCP+ at-risk subjects as compared to CCP- healthy subjects in a cross-sectional cohort. We now extend this study utilizing a longitudinal cohort including HLA-DRB1*0401 CCP- healthy subjects, CCP+ at-risk subjects, RA converters (at-risk individuals sampled just prior to transitioning to RA), and patients with new-onset RA.
Methods: Using DRB1*0401 tetramers containing known citrullinated T cell peptide antigens, we assessed the frequency and surface phenotype of cit-specific CD4+ T cells. To determine the TCR sequences and transcriptome of antigen-specific CD4+ T cells, we also performed Antigen-specific T cell sequencing (Ag-Tseq), which combines robust methods for isolation and enrichment of antigen-specific CD4+ T cells with single-cell RNA-seq (scRNA-seq).
Results: Cit-specific T cells were detected in individuals from all four groups and the frequency of total antigen-specific CD4+ T cells was stable in single individuals over time in each cohort. Notably, CCP+ RA converters had lower frequencies of circulating antigen specific CD4+ T cells than CCP+ at-risk subjects. Additionally, we observed a multiplicity of phenotypes with no single dominant lineage based on antigen specificity, individual subject or disease state. We performed Ag-Tseq on cit-antigen-specific-CD4+ T cells from CCP+ at-risk subjects (n=6), subjects with new onset RA (n=6) and an RA converter (n=1). UMAP analysis identified six distinct clusters (Figure 1). Vimentin- and fibrinogen-specific cells were predominantly found in a cluster dominant for IL7R and ANXA1, CILP-specific cells were found in the naive-like cluster and the activated clusters, whereas enolase-specific cells were present in multiple clusters. Expanded TCR clonotypes from cit-specific CD4+ T cells in CCP+ at-risk, new-onset RA and RA converter subjects were identified at individual time points, some of which were maintained at later time points (Figure 2). To verify the specificity of the expanded TCR clonotypes, we transduced primary human CD4+ T cells with lentivirus expressing the TCR and confirmed activation and expansion of cit-CILP108 TCRs in an at-risk subject. Therefore, cells with this TCR persisted, and were activated and expanded in vivo. This is consistent with our prior study which demonstrated that cit-CILP epitopes were the dominant specificity in CCP+ subjects, which is distinct from new-onset RA.
Conclusion: Cit-specific CD4+ T cells are present and exhibit diverse specificities and cell subset inclusion in both established RA and prior to arthritis development and RA classification. Our findings indicate that some cit-specific CD4+ T cells are activated and expanded in at-risk individuals. This work suggests that the study of these antigen-specific T cell populations may identify novel immune pathways to target therapeutically in RA.
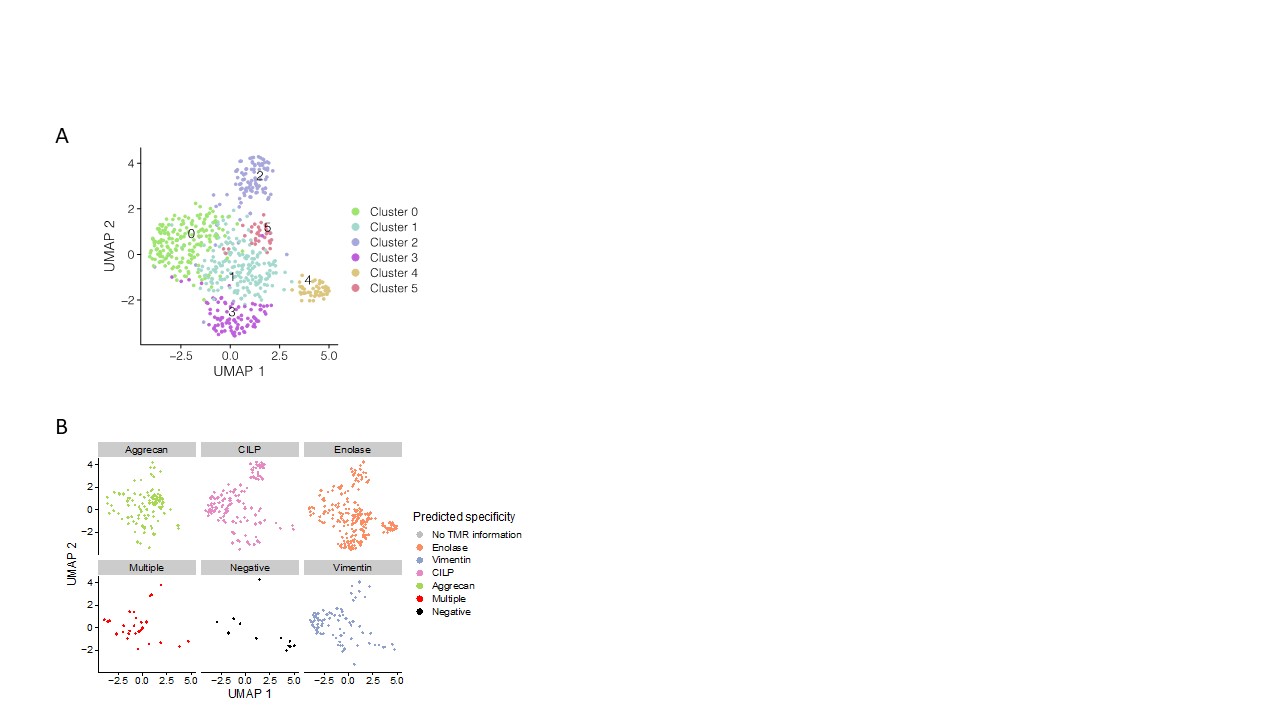 Figure 1. A. RA antigen specific cells form 6 clusters using UMAP. B. Antigen-specific cells from Ag-Tseq locate in different clusters.
Figure 1. A. RA antigen specific cells form 6 clusters using UMAP. B. Antigen-specific cells from Ag-Tseq locate in different clusters.
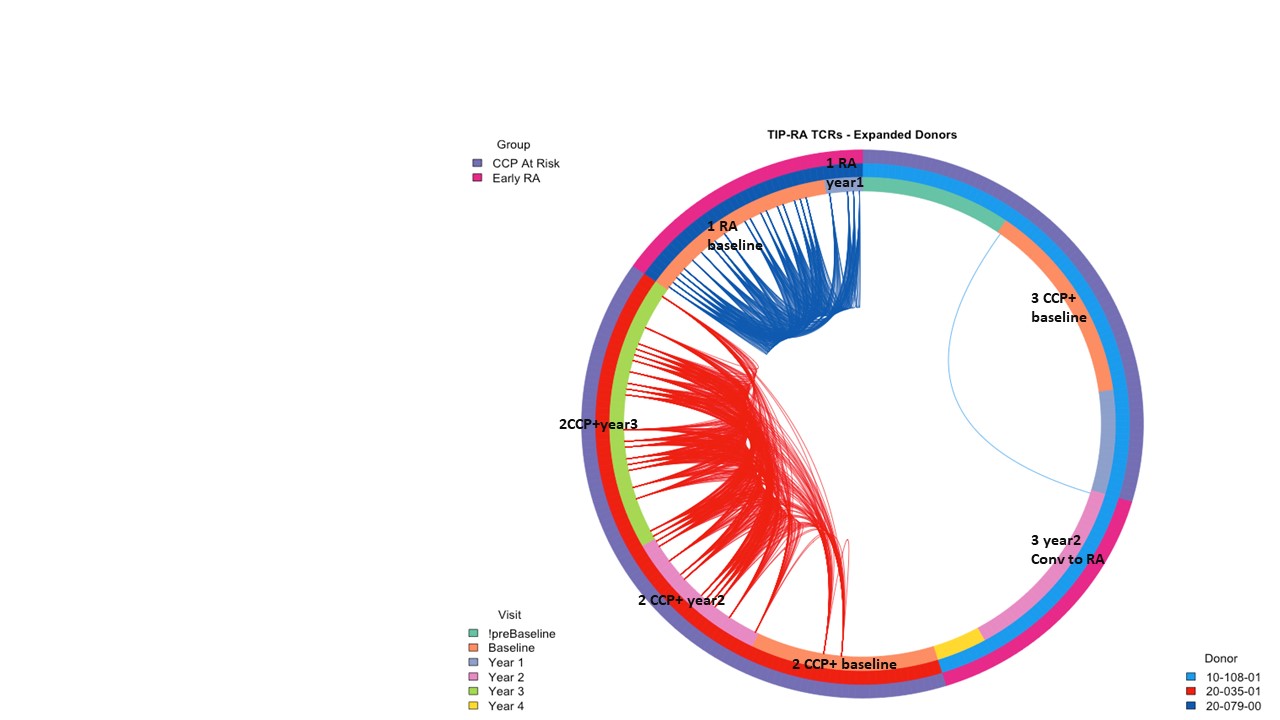 Figure 2. Private shared TCRs persist over time in CCP+ at risk, RA converter and early RA subject.
Figure 2. Private shared TCRs persist over time in CCP+ at risk, RA converter and early RA subject.
Disclosures: J. Song, None; S. Presnell, None; C. Rims, None; M. Dufort, None; H. DeBerg, None; P. Linsley, None; V. Holers, Janssen; K. Deane, Werfen; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors.
Background/Purpose: Rheumatoid arthritis (RA) is an autoimmune disease, in which autoantibodies targeting citrullinated (cit) peptides can be detected prior to diagnosis. We previously reported an increase in cit-specific CD4+ T cells in HLA-DRB1*0401 CCP+ at-risk subjects as compared to CCP- healthy subjects in a cross-sectional cohort. We now extend this study utilizing a longitudinal cohort including HLA-DRB1*0401 CCP- healthy subjects, CCP+ at-risk subjects, RA converters (at-risk individuals sampled just prior to transitioning to RA), and patients with new-onset RA.
Methods: Using DRB1*0401 tetramers containing known citrullinated T cell peptide antigens, we assessed the frequency and surface phenotype of cit-specific CD4+ T cells. To determine the TCR sequences and transcriptome of antigen-specific CD4+ T cells, we also performed Antigen-specific T cell sequencing (Ag-Tseq), which combines robust methods for isolation and enrichment of antigen-specific CD4+ T cells with single-cell RNA-seq (scRNA-seq).
Results: Cit-specific T cells were detected in individuals from all four groups and the frequency of total antigen-specific CD4+ T cells was stable in single individuals over time in each cohort. Notably, CCP+ RA converters had lower frequencies of circulating antigen specific CD4+ T cells than CCP+ at-risk subjects. Additionally, we observed a multiplicity of phenotypes with no single dominant lineage based on antigen specificity, individual subject or disease state. We performed Ag-Tseq on cit-antigen-specific-CD4+ T cells from CCP+ at-risk subjects (n=6), subjects with new onset RA (n=6) and an RA converter (n=1). UMAP analysis identified six distinct clusters (Figure 1). Vimentin- and fibrinogen-specific cells were predominantly found in a cluster dominant for IL7R and ANXA1, CILP-specific cells were found in the naive-like cluster and the activated clusters, whereas enolase-specific cells were present in multiple clusters. Expanded TCR clonotypes from cit-specific CD4+ T cells in CCP+ at-risk, new-onset RA and RA converter subjects were identified at individual time points, some of which were maintained at later time points (Figure 2). To verify the specificity of the expanded TCR clonotypes, we transduced primary human CD4+ T cells with lentivirus expressing the TCR and confirmed activation and expansion of cit-CILP108 TCRs in an at-risk subject. Therefore, cells with this TCR persisted, and were activated and expanded in vivo. This is consistent with our prior study which demonstrated that cit-CILP epitopes were the dominant specificity in CCP+ subjects, which is distinct from new-onset RA.
Conclusion: Cit-specific CD4+ T cells are present and exhibit diverse specificities and cell subset inclusion in both established RA and prior to arthritis development and RA classification. Our findings indicate that some cit-specific CD4+ T cells are activated and expanded in at-risk individuals. This work suggests that the study of these antigen-specific T cell populations may identify novel immune pathways to target therapeutically in RA.
 Figure 1. A. RA antigen specific cells form 6 clusters using UMAP. B. Antigen-specific cells from Ag-Tseq locate in different clusters.
Figure 1. A. RA antigen specific cells form 6 clusters using UMAP. B. Antigen-specific cells from Ag-Tseq locate in different clusters. Figure 2. Private shared TCRs persist over time in CCP+ at risk, RA converter and early RA subject.
Figure 2. Private shared TCRs persist over time in CCP+ at risk, RA converter and early RA subject.Disclosures: J. Song, None; S. Presnell, None; C. Rims, None; M. Dufort, None; H. DeBerg, None; P. Linsley, None; V. Holers, Janssen; K. Deane, Werfen; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors.

