Back
Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – ANCA-Associated (0524–0529)
0525: Recovery of Renal Function Among ANCA-Associated Vasculitis Patients with Baseline eGFR ≤20 in the Avacopan ADVOCATE Trial
Saturday, November 12, 2022
3:15 PM – 3:25 PM Eastern Time
Location: Room 103
- FC
Presenting Author(s)
David Jayne1, Peter Merkel2, Frank Cortazar3, John Niles4 and Pirow Bekker5, 1University of Cambridge, Cambridge, United Kingdom, 2U of Pennsylvania, Philadelphia, PA, 3St. Peter's Hospital, Albany, NY, 4Harvard, Boston, MA, 5ChemoCentryx, San Juan Capistrano, CA
Background/Purpose: In the 330-patient ADVOCATE trial of avacopan for the treatment of ANCA-associated vasculitis, in which 81% of patients with ANCA-associated vasculitis had renal involvement, estimated glomerular filtration rate (eGFR) increased on average 7.3 mL/min/1.73 m2 in the avacopan group and 4.1 in the prednisone group (P=0.029) at Week 52 (Jayne et al. 2021). The aim of this post hoc analysis was to evaluate changes in eGFR in patients in ADVOCATE who upon enrolment into the trial approached the threshold for dialysis, i.e., eGFR ≤20 mL/min/1.73 m2.
Methods: eGFR (based on serum creatinine and the Modification of Diet in Renal Disease equation) was measured at baseline and over the course of the trial. Data from patients in ADVOCATE with an eGFR ≤20 mL/min/1.73 m2 at baseline were included in this analysis.
Results: In ADVOCATE, 27 of 166 patients (16%) in the avacopan group and 23 of 164 patients (14%) in the prednisone group had a baseline eGFR ≤20 mL/min/1.73 m2. Baseline characteristics were similar across treatment groups (Table 1). The mean age in this low eGFR group of 50 patients was similar to the overall 330-patient population (66 vs. 61 years), but included a higher proportion of patients with newly diagnosed disease (88 vs. 69%), MPO+ (84 vs. 57%), and MPA (72 vs. 45%), and higher use of cyclophosphamide (50 vs. 35%).
Individual eGFR data from all patients are shown in Figure 1. eGFR increased on average 16.1 and 7.7 mL/min/1.73 m2 at Week 52 in the avacopan and prednisone groups, respectively (P=0.003). The last eGFR value measured during the 52-week treatment period was >20 mL/min/1.73 m2 in 85% of patients in the avacopan group compared to 57% of patients in the prednisone group (P=0.024). 41% of patients in the avacopan group had an increase in eGFR of ≥2-fold vs. 13% in the prednisone group (P=0.030). A higher number of patients in the avacopan group had increases in eGFR above 30 and 45 mL/min/1.73 m2, respectively. eGFR in one patient in the avacopan group increased to 65 at Week 52 (baseline 17). Urinary albumin:creatinine ratio levels improved more rapidly in the avacopan vs. prednisone group. Serious adverse events occurred in 13/27 patients (48%) in the avacopan group (1 death due to bronchopneumonia) and 16/23 patients (70%) in the prednisone group (1 death due pleural empyema).
Conclusion: Among patients with baseline eGFR ≤20 mL/min/1.73 m2 in the ADVOCATE trial, eGFR improved more in the avacopan vs. control group.
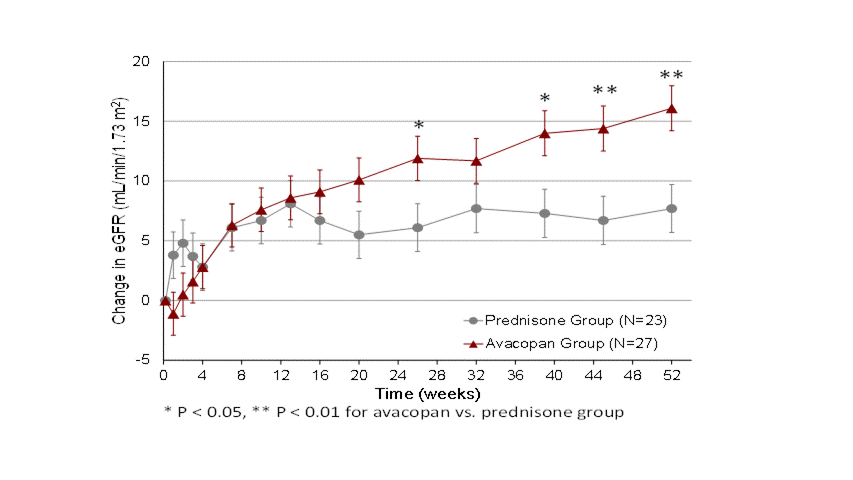 Figure 1: Change from Baseline in eGFR by Treatment Group over the Course of the 52-Week Treatment Period in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
Figure 1: Change from Baseline in eGFR by Treatment Group over the Course of the 52-Week Treatment Period in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
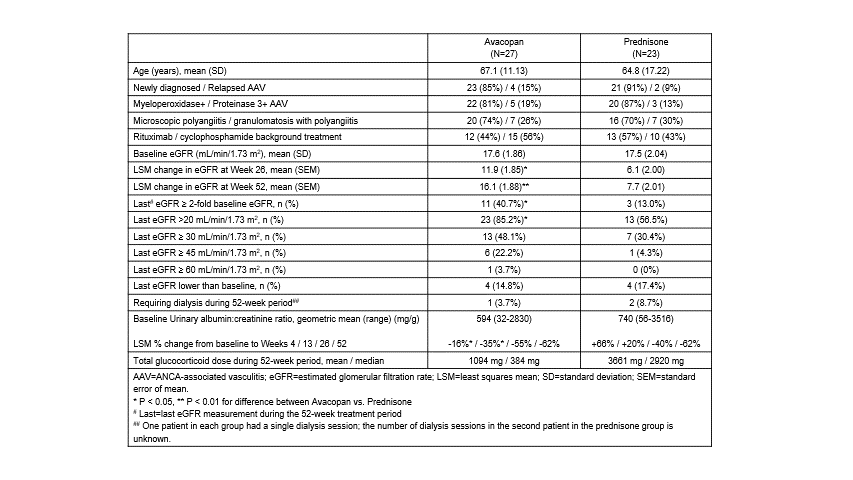 Table 1: Summary of Data on eGFR in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
Table 1: Summary of Data on eGFR in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
Disclosures: D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; P. Merkel, ChemoCentryx; F. Cortazar, ChemoCentryx; J. Niles, ChemoCentryx; P. Bekker, Chemocentryx.
Background/Purpose: In the 330-patient ADVOCATE trial of avacopan for the treatment of ANCA-associated vasculitis, in which 81% of patients with ANCA-associated vasculitis had renal involvement, estimated glomerular filtration rate (eGFR) increased on average 7.3 mL/min/1.73 m2 in the avacopan group and 4.1 in the prednisone group (P=0.029) at Week 52 (Jayne et al. 2021). The aim of this post hoc analysis was to evaluate changes in eGFR in patients in ADVOCATE who upon enrolment into the trial approached the threshold for dialysis, i.e., eGFR ≤20 mL/min/1.73 m2.
Methods: eGFR (based on serum creatinine and the Modification of Diet in Renal Disease equation) was measured at baseline and over the course of the trial. Data from patients in ADVOCATE with an eGFR ≤20 mL/min/1.73 m2 at baseline were included in this analysis.
Results: In ADVOCATE, 27 of 166 patients (16%) in the avacopan group and 23 of 164 patients (14%) in the prednisone group had a baseline eGFR ≤20 mL/min/1.73 m2. Baseline characteristics were similar across treatment groups (Table 1). The mean age in this low eGFR group of 50 patients was similar to the overall 330-patient population (66 vs. 61 years), but included a higher proportion of patients with newly diagnosed disease (88 vs. 69%), MPO+ (84 vs. 57%), and MPA (72 vs. 45%), and higher use of cyclophosphamide (50 vs. 35%).
Individual eGFR data from all patients are shown in Figure 1. eGFR increased on average 16.1 and 7.7 mL/min/1.73 m2 at Week 52 in the avacopan and prednisone groups, respectively (P=0.003). The last eGFR value measured during the 52-week treatment period was >20 mL/min/1.73 m2 in 85% of patients in the avacopan group compared to 57% of patients in the prednisone group (P=0.024). 41% of patients in the avacopan group had an increase in eGFR of ≥2-fold vs. 13% in the prednisone group (P=0.030). A higher number of patients in the avacopan group had increases in eGFR above 30 and 45 mL/min/1.73 m2, respectively. eGFR in one patient in the avacopan group increased to 65 at Week 52 (baseline 17). Urinary albumin:creatinine ratio levels improved more rapidly in the avacopan vs. prednisone group. Serious adverse events occurred in 13/27 patients (48%) in the avacopan group (1 death due to bronchopneumonia) and 16/23 patients (70%) in the prednisone group (1 death due pleural empyema).
Conclusion: Among patients with baseline eGFR ≤20 mL/min/1.73 m2 in the ADVOCATE trial, eGFR improved more in the avacopan vs. control group.
 Figure 1: Change from Baseline in eGFR by Treatment Group over the Course of the 52-Week Treatment Period in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
Figure 1: Change from Baseline in eGFR by Treatment Group over the Course of the 52-Week Treatment Period in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2 Table 1: Summary of Data on eGFR in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2
Table 1: Summary of Data on eGFR in the ADVOCATE trial of Avacopan for ANCA-associated Vasculitis in Patients with Baseline eGFR ≤20 mL/min/1.73 m2Disclosures: D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; P. Merkel, ChemoCentryx; F. Cortazar, ChemoCentryx; J. Niles, ChemoCentryx; P. Bekker, Chemocentryx.

