Back
Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – ANCA-Associated (0524–0529)
0526: Characteristics and Outcomes of Participants with and Without Diffuse Alveolar Hemorrhage in the Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis (PEXIVAS) Trial
Saturday, November 12, 2022
3:30 PM – 3:40 PM Eastern Time
Location: Room 103
- LF
Lynn Fussner, MD
The Ohio State University
Columbus, OH, United States
Presenting Author(s)
Lynn Fussner1, Luis Felipe Flores-Suarez2, Rodrigo Cartin-Ceba3, Ulrich Specks3, P. Gerard Cox4, David Jayne5, Peter Merkel6 and Michael Walsh4, 1The Ohio State University, Columbus, OH, 2Instituto Nacional de Enfermedades Respiratorias, Ciudad de México, Mexico, 3Mayo Clinic, Rochester, MN, 4McMaster University, Hamilton, ON, Canada, 5University of Cambridge, Cambridge, United Kingdom, 6University of Pennsylvania, Philadelphia, PA
Background/Purpose: Diffuse alveolar hemorrhage (DAH) is a potentially life-threatening manifestation of ANCA-associated vasculitis (AAV). Studies describing patients with DAH in AAV have typically been small and/or retrospective. Presented here are the baseline and outcome data for participants with and without DAH in the Plasma Exchange (PLEX) and Glucocorticoids (GC) in Severe ANCA-Associated Vasculitis (PEXIVAS) trial.
Methods: Clinical data were collected prospectively from 704 participants with AAV at 95 centers in 16 countries in PEXIVAS, a randomized controlled trial comparing 7 sessions of PLEX vs. none, along with two GC regimens. For the present analysis, treatment groups were combined and participants grouped based on presence or absence of DAH at enrollment. Baseline characteristics and one year outcomes are summarized and compared. Participants with DAH were, a priori, subcategorized as having severe DAH if they had oxygen saturation ≤85% on room air or were on mechanical ventilation at enrollment.
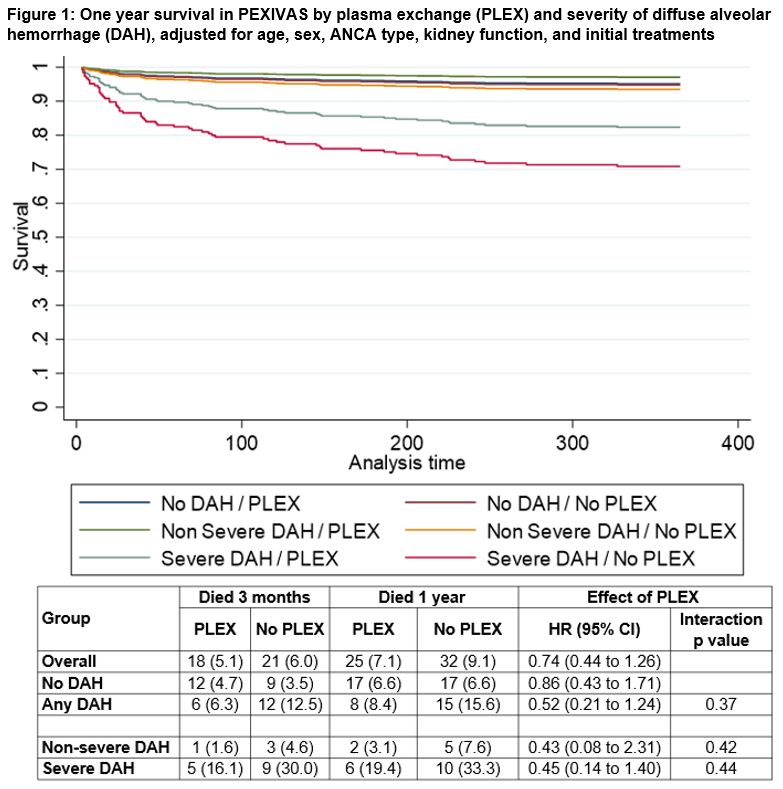
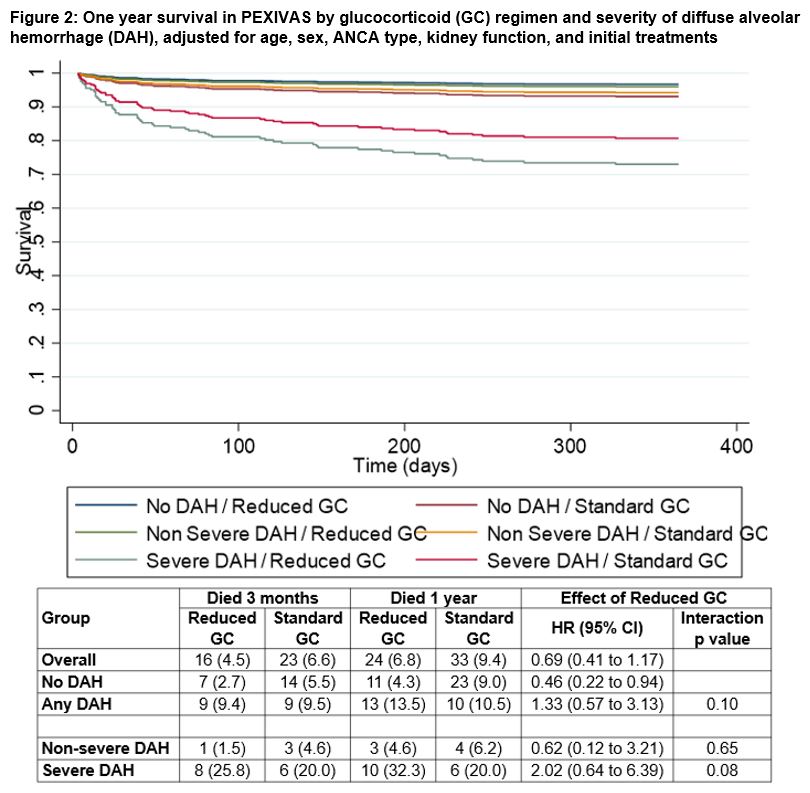
Results: Among the 704 participants in PEXIVAS, 191 (27%) had DAH. Participants with DAH were younger (mean age 61.1 years with AH vs. 63.9 years without DAH), more frequently proteinase 3-ANCA positive (51.5% vs. 36.5%), and more frequently had relapsing AAV (as opposed to a new diagnosis) at enrollment (14.1% vs. 7.0%). Participants with DAH had higher baseline disease severity based on BVAS-WG (median 11 vs. 7) and lower serum creatinine (median 184 vs. 304 µmol/L) but were more frequently dialyzed at enrollment (25.3% vs. 18.0%). Additional baseline characteristics are presented in Table 1. Among the 191 participants with DAH, 61 (31.9%) had severe DAH, of which 29 (15.2%) required mechanical ventilation. Twenty-three (12.0%) participants with DAH at enrollment died in the first year as compared to 34 (6.6%) without DAH. Among participants who died within one year of enrollment, 14 of 23 (60.9%) deaths among participants with DAH occurred within 30 days of enrollment vs. 11 of 34 (32.4%) in those without DAH. End-stage kidney disease (ESKD) and serious infections in the first year were similar between participants with and without DAH (ESKD 16.0% vs. 13.1%; serious infections 41.4% vs. 39.2%). There was no evidence of variation in the treatment effects of PLEX (Figure 1, p-interaction=0.37) or GC treatment arm (Figure 2, p-interaction=0.10) between those who did and did not have DAH at enrollment.
Conclusion: Based on the PEXIVAS trial cohort, patients with AAV and DAH differ from those without DAH in multiple other ways, including age, ANCA specificity, and use of dialysis. Patients with DAH are more likely to die within 30 days and one year than patients without DAH. These results underscore the need to identify safe and effective therapies for patients with severe DAH.
.jpg)
Disclosures: L. Fussner, None; L. Flores-Suarez, None; R. Cartin-Ceba, None; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; P. Cox, None; D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate; M. Walsh, Bayer, Vifor, Novo Nordisc, Otsuka.
Background/Purpose: Diffuse alveolar hemorrhage (DAH) is a potentially life-threatening manifestation of ANCA-associated vasculitis (AAV). Studies describing patients with DAH in AAV have typically been small and/or retrospective. Presented here are the baseline and outcome data for participants with and without DAH in the Plasma Exchange (PLEX) and Glucocorticoids (GC) in Severe ANCA-Associated Vasculitis (PEXIVAS) trial.
Methods: Clinical data were collected prospectively from 704 participants with AAV at 95 centers in 16 countries in PEXIVAS, a randomized controlled trial comparing 7 sessions of PLEX vs. none, along with two GC regimens. For the present analysis, treatment groups were combined and participants grouped based on presence or absence of DAH at enrollment. Baseline characteristics and one year outcomes are summarized and compared. Participants with DAH were, a priori, subcategorized as having severe DAH if they had oxygen saturation ≤85% on room air or were on mechanical ventilation at enrollment.
Results: Among the 704 participants in PEXIVAS, 191 (27%) had DAH. Participants with DAH were younger (mean age 61.1 years with AH vs. 63.9 years without DAH), more frequently proteinase 3-ANCA positive (51.5% vs. 36.5%), and more frequently had relapsing AAV (as opposed to a new diagnosis) at enrollment (14.1% vs. 7.0%). Participants with DAH had higher baseline disease severity based on BVAS-WG (median 11 vs. 7) and lower serum creatinine (median 184 vs. 304 µmol/L) but were more frequently dialyzed at enrollment (25.3% vs. 18.0%). Additional baseline characteristics are presented in Table 1. Among the 191 participants with DAH, 61 (31.9%) had severe DAH, of which 29 (15.2%) required mechanical ventilation. Twenty-three (12.0%) participants with DAH at enrollment died in the first year as compared to 34 (6.6%) without DAH. Among participants who died within one year of enrollment, 14 of 23 (60.9%) deaths among participants with DAH occurred within 30 days of enrollment vs. 11 of 34 (32.4%) in those without DAH. End-stage kidney disease (ESKD) and serious infections in the first year were similar between participants with and without DAH (ESKD 16.0% vs. 13.1%; serious infections 41.4% vs. 39.2%). There was no evidence of variation in the treatment effects of PLEX (Figure 1, p-interaction=0.37) or GC treatment arm (Figure 2, p-interaction=0.10) between those who did and did not have DAH at enrollment.
Conclusion: Based on the PEXIVAS trial cohort, patients with AAV and DAH differ from those without DAH in multiple other ways, including age, ANCA specificity, and use of dialysis. Patients with DAH are more likely to die within 30 days and one year than patients without DAH. These results underscore the need to identify safe and effective therapies for patients with severe DAH.
.jpg)


Disclosures: L. Fussner, None; L. Flores-Suarez, None; R. Cartin-Ceba, None; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; P. Cox, None; D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate; M. Walsh, Bayer, Vifor, Novo Nordisc, Otsuka.

