Back
Poster Session D
Systemic lupus erythematosus (SLE)
Session: (1707–1726) SLE – Animal Models Poster
1707: Exploring the Role of Lipocalin-2 in Neuropsychiatric SLE
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SG
Sayra Garcia, MS, BS
Albert Einstein College of Medicine
New York, NY, United States
Abstract Poster Presenter(s)
Chaim Putterman1, Sayra Garcia-Murillo2 and Elise Mike3, 1Albert Einstein College of Medicine, Bronx, NY, 2Albert Einstein College of Medicine, New York, NY, 3John Hopkins University, Baltimore, MD
Background/Purpose: While the etiology of neuropsychiatric lupus (NPSLE) is not fully understood, blood brain barrier (BBB) disruption and localized neuroinflammation are potential mechanisms that contribute to disease progression. Lipocalin-2 (LCN2) is a multi-functional acute phase protein known to affect immune cell function, BBB integrity, and glial cell activation. The MRL/lpr mouse strain not only captures multiple human facets of SLE such as female predominance, nephritis, and cutaneous disease, it is the mostly widely used NPSLE model. Displaying cognitive deficits and depression-like behavior at an early age, the MRL/lpr mouse is an important model for studying NPSLE disease mechanisms. We generated an LCN2-deficient MRL/lpr mouse and examined the effects of LCN2 deficiency on neurobehavioral parameters, uncovering a role of LCN2 in cognitive and affective deficits in the MRL/lpr strain.
Methods: Behavioral assessment was performed on LCN2 deficient MRL/lpr mice (LCN2KO), MRL/lpr LCN2 sufficient mice, and MRL/MpJ mice at 16 weeks of age. The tests performed include the behavioral spectrometer, anhedonia, tail suspension, Porsolt forced swim, object placement, and object recognition tests. The mice were sacrificed promptly after completion of the neurobehavioral assessments and tissues were either snap frozen for RNA and protein studies, or fixed in paraformaldehyde for immunofluorescent staining. Systemic disease was evaluated through monitoring anti-nuclear antibodies, total IgG levels, and kidney disease, and as well as lymph node size and the severity of skin disease. To evaluate how LCN2 deficiency affects brain inflammation, 10 week old LCN2KO and MRL/lpr mice were injected intraperitoneally with 5 mg/kg lipopolysaccharide. The mice were then sacrificed, perfused, and the brains were fixed and paraffin-embedded for immunofluorescent staining. Brain tissue was stained for infiltrating leukocytes as well as glial cells and LCN2 protein.
Results: We found that LCN2 deficiency in MRL/lpr mice did not affect serum anti-nuclear antibody, serum IgG, or proteinuria as shown by ELISA. However, cutaneous disease was significantly improved in LCN2-deficient MRL/lpr mice (Figure 1). The behavioral analyses additionally showed significant improvement in LCN2 deficient mice, in the tail suspension (not shown), Porsolt forced swim, and object placement tests (Figure 2). Finally, following an inflammatory stimulus, LCN2 deficiency resulted in significantly decreased activation of astrocytes in the hippocampus, a brain region involved in emotionality and cognitive function.
Conclusion: Our results point to an important role of LCN2 in perpetuating local brain inflammation, that may contribute to depression and cognitive deficits in MRL/lpr mice. LCN2 deficiency not only improves the NPSLE phenotype in this strain, but also decreases local brain inflammation. Since kidney disease and serologic features were largely unaffected, it is likely that LCN2 plays a role more locally in the brain, suggesting LCN2 as a potential target for the treatment of NPSLE in patients who do not successfully respond to traditional therapies for diffuse disease.
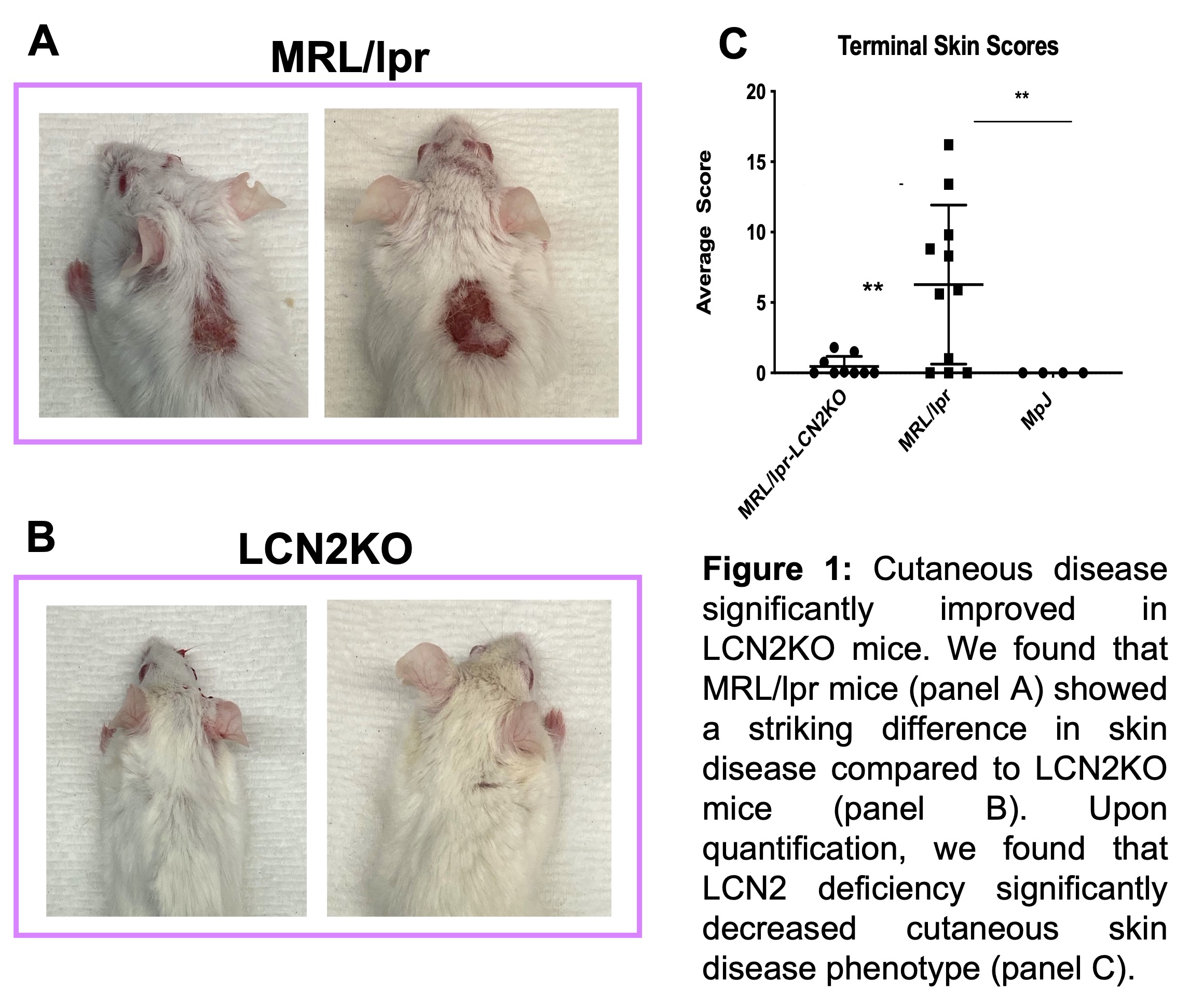
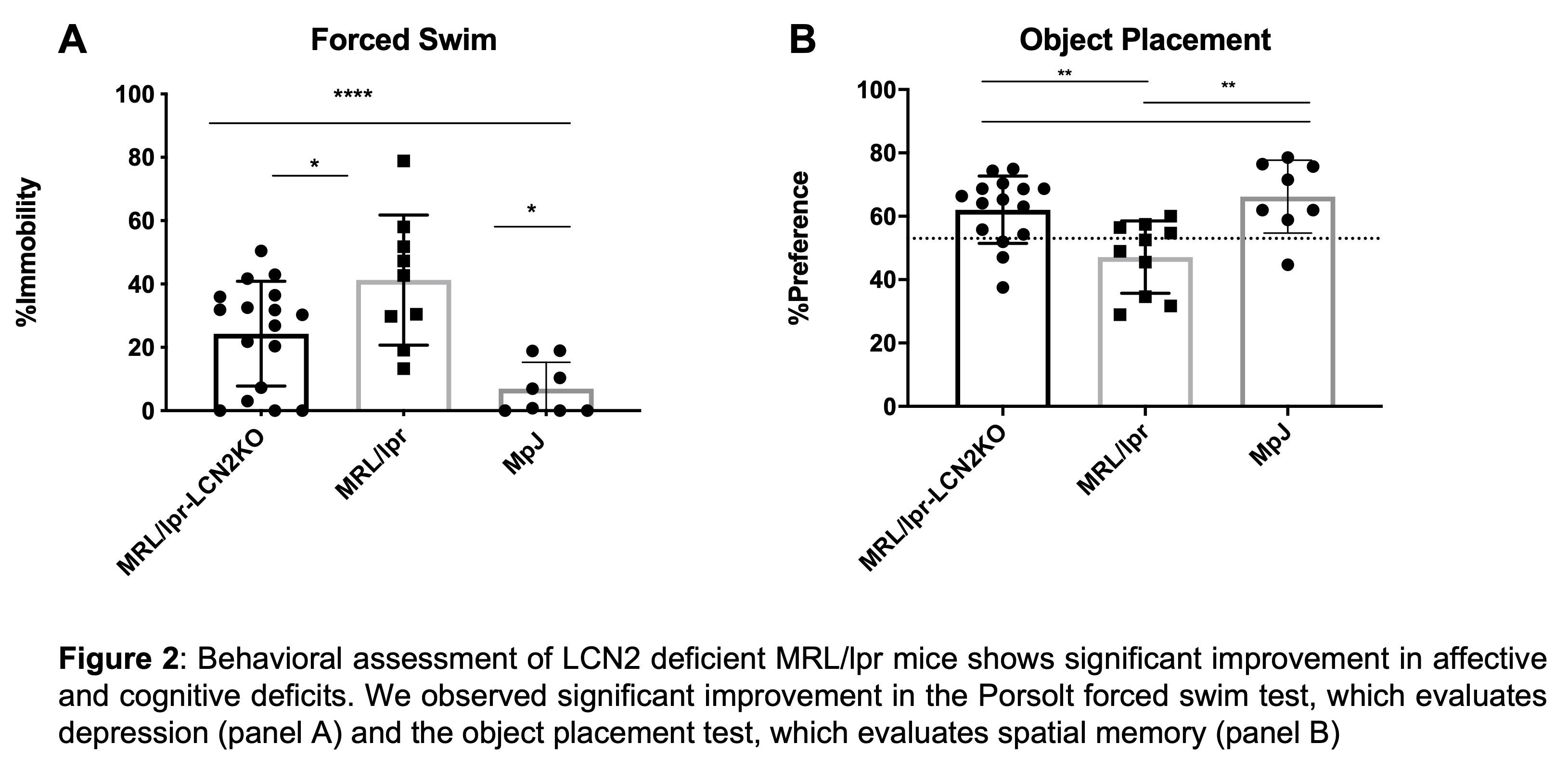
Disclosures: C. Putterman, Equillium, Progentec, Kidneycure; S. Garcia-Murillo, None; E. Mike, None.
Background/Purpose: While the etiology of neuropsychiatric lupus (NPSLE) is not fully understood, blood brain barrier (BBB) disruption and localized neuroinflammation are potential mechanisms that contribute to disease progression. Lipocalin-2 (LCN2) is a multi-functional acute phase protein known to affect immune cell function, BBB integrity, and glial cell activation. The MRL/lpr mouse strain not only captures multiple human facets of SLE such as female predominance, nephritis, and cutaneous disease, it is the mostly widely used NPSLE model. Displaying cognitive deficits and depression-like behavior at an early age, the MRL/lpr mouse is an important model for studying NPSLE disease mechanisms. We generated an LCN2-deficient MRL/lpr mouse and examined the effects of LCN2 deficiency on neurobehavioral parameters, uncovering a role of LCN2 in cognitive and affective deficits in the MRL/lpr strain.
Methods: Behavioral assessment was performed on LCN2 deficient MRL/lpr mice (LCN2KO), MRL/lpr LCN2 sufficient mice, and MRL/MpJ mice at 16 weeks of age. The tests performed include the behavioral spectrometer, anhedonia, tail suspension, Porsolt forced swim, object placement, and object recognition tests. The mice were sacrificed promptly after completion of the neurobehavioral assessments and tissues were either snap frozen for RNA and protein studies, or fixed in paraformaldehyde for immunofluorescent staining. Systemic disease was evaluated through monitoring anti-nuclear antibodies, total IgG levels, and kidney disease, and as well as lymph node size and the severity of skin disease. To evaluate how LCN2 deficiency affects brain inflammation, 10 week old LCN2KO and MRL/lpr mice were injected intraperitoneally with 5 mg/kg lipopolysaccharide. The mice were then sacrificed, perfused, and the brains were fixed and paraffin-embedded for immunofluorescent staining. Brain tissue was stained for infiltrating leukocytes as well as glial cells and LCN2 protein.
Results: We found that LCN2 deficiency in MRL/lpr mice did not affect serum anti-nuclear antibody, serum IgG, or proteinuria as shown by ELISA. However, cutaneous disease was significantly improved in LCN2-deficient MRL/lpr mice (Figure 1). The behavioral analyses additionally showed significant improvement in LCN2 deficient mice, in the tail suspension (not shown), Porsolt forced swim, and object placement tests (Figure 2). Finally, following an inflammatory stimulus, LCN2 deficiency resulted in significantly decreased activation of astrocytes in the hippocampus, a brain region involved in emotionality and cognitive function.
Conclusion: Our results point to an important role of LCN2 in perpetuating local brain inflammation, that may contribute to depression and cognitive deficits in MRL/lpr mice. LCN2 deficiency not only improves the NPSLE phenotype in this strain, but also decreases local brain inflammation. Since kidney disease and serologic features were largely unaffected, it is likely that LCN2 plays a role more locally in the brain, suggesting LCN2 as a potential target for the treatment of NPSLE in patients who do not successfully respond to traditional therapies for diffuse disease.


Disclosures: C. Putterman, Equillium, Progentec, Kidneycure; S. Garcia-Murillo, None; E. Mike, None.

