Back
Abstract Session
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics (0518–0523)
0521: Examination of Differential Response to Treatment with Mycophenolate Mofetil in Black and White Patients with Systemic Sclerosis
Saturday, November 12, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 121
- LS
Lauren Smith, MD
Johns Hopkins
District Heights, MD, United States
Presenting Author(s)
Lauren Smith1, Jamie Perin2, Adrianne Woods2, Fredrick Wigley3, Laura Hummers4 and Ami Shah5, 1Johns Hopkins, District Heights, MD, 2John Hopkins University, Baltimore, MD, 3Johns Hopkins University, Baltimore, MD, 4Johns Hopkins Univerisity, Baltimore, MD, 5Johns Hopkins Rheumatology, Baltimore, MD
Background/Purpose: Black patients with systemic sclerosis (SSc) develop SSc at a younger age and have more severe disease than White patients, including a higher prevalence of diffuse cutaneous SSc (dcSSc) and interstitial lung disease (ILD). We sought to harness data from a large, diverse longitudinal prospective cohort to investigate whether Black and White scleroderma patients have different therapeutic responses to mycophenolate mofetil.
Methods: Self-identified Black and White patients with SSc who had exposure to mycophenolate mofetil (MMF) were selected from the Johns Hopkins Scleroderma Center (JHSC) Research Registry. Patients without at least one PFT and mRSS measurement prior to or within one month of initiation of MMF therapy were excluded. We restricted our study population to patients who presented to our Center within 5 years of disease onset. Our primary outcome measures were absolute change in forced vital capacity (FVC) % predicted and modified Rodnan skin scores (mRSS) over a 5-year period.
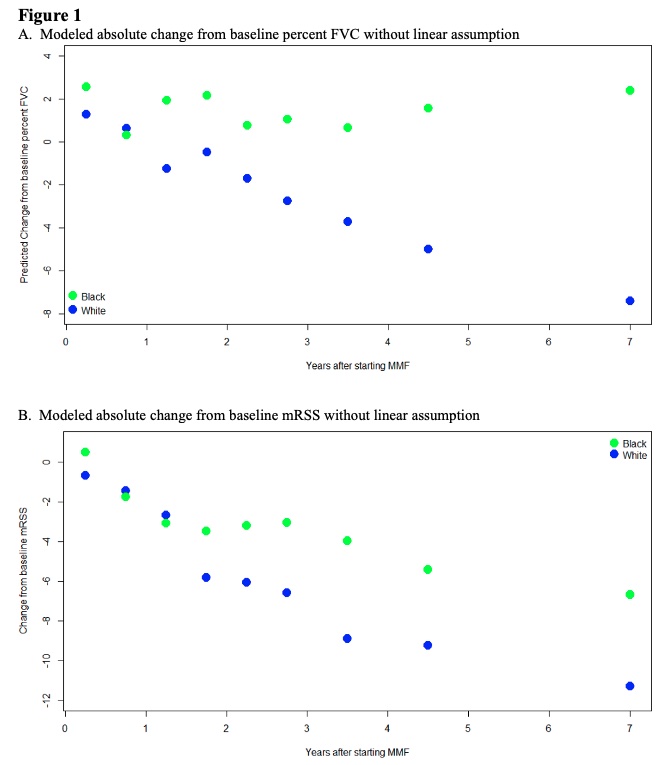
Results: 718 patients with SSc who self-identified as either Black (n=152) or White (n=566) were exposed to MMF. Black patients were younger at SSc onset than White patients (mean (SD) 40.1 (13.3) years vs. 47.3 (13.1)), though both groups presented to our Center at a similar disease duration (Table 1). Both groups were comparable in terms of sex and proportion of patients with diffuse cutaneous disease. The most common autoantibody was anti-Scl-70 (46%) for Black patients and anti-RNA polymerase III (46%) for White patients. The majority of subjects in both groups achieved the maximum dose of MMF (3g) for at least 3 months (55% for Black patients, 52% for White patients). In the cohort of patients who had baseline and follow up FVC, the average baseline FVC% predicted was 66.7 (20.0) for Black patients and 83.2 (19.0) for White patients prior to treatment with MMF (Table 2). At 1 year, the modeled absolute change in FVC% predicted for Black patients was +1.7 (1.7) compared to -1.2 (0.7) for White patients (p< 0.001). By year 5, the modeled absolute change in FVC% predicted for Black patients was +1.7 (1.7) compared to -4.9 (0.7) for White patients (p< 0.001). Among patients with dcSSc, the baseline mRSS was 13.3 (11.0) for Black patients and 17.7 (13.7) for White patients (p=0.212). At 1 year, the modeled absolute change in mRSS from baseline was -3.1(1.7) for Black patients and -3.4(1.3) for White patients (p=0.907). By year 5, the modeled absolute change in mRSS for Black patients was -5.4 (1.9) compared to -9.8 (1.3) for White patients (p=0.133).
Conclusion: There is a significant difference in baseline FVC% predicted between Black and White patients, but once treated with MMF, Black patients' FVC% predicted appears to stabilize while White patients have a slow decline. Both groups show a similar reduction in mRSS skin scores with treatment of MMF. Overall, these results suggest that worse pulmonary outcomes for Black patients are not due to reduced therapeutic efficacy but may be due to more severe disease at presentation. Ongoing work is needed to investigate if there is a biologic basis for this differential disease severity and the role of social determinants of health as predictors of worse outcomes in Black versus White SSc patients.
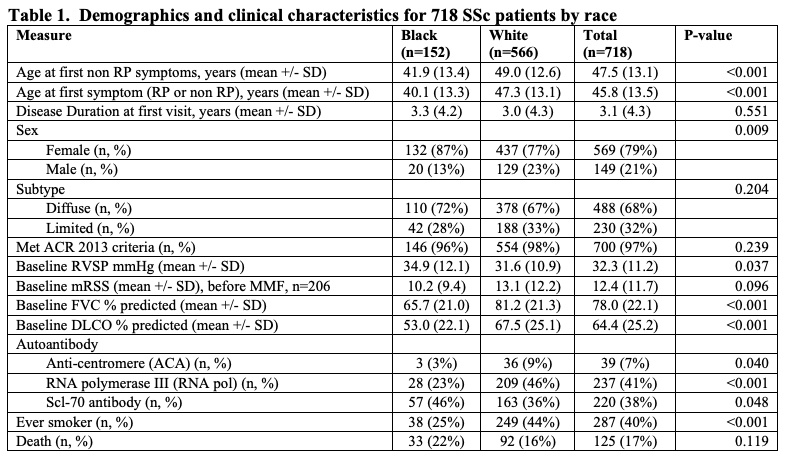 Table 1. Demographics and clinical characteristics for 718 SSc patients by race
Table 1. Demographics and clinical characteristics for 718 SSc patients by race
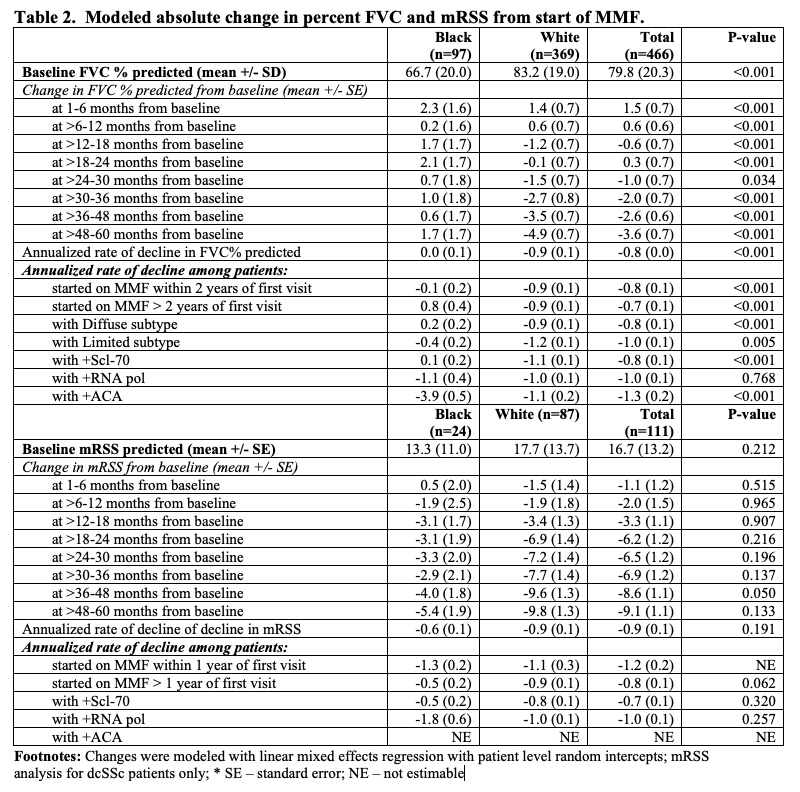 Table 2. Modeled absolute change in percent FVC and mRSS from start of MMF.
Table 2. Modeled absolute change in percent FVC and mRSS from start of MMF.
Disclosures: L. Smith, None; J. Perin, None; A. Woods, None; F. Wigley, None; L. Hummers, Boehringer-Ingelheim, Corbus Pharmaceuticals, Cumberland Pharmaceuticals, Kadmon Corporation, Medpace, CSL Behring, Mitsubishi Tanabe, Horizon Pharmaceuticals; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation.
Background/Purpose: Black patients with systemic sclerosis (SSc) develop SSc at a younger age and have more severe disease than White patients, including a higher prevalence of diffuse cutaneous SSc (dcSSc) and interstitial lung disease (ILD). We sought to harness data from a large, diverse longitudinal prospective cohort to investigate whether Black and White scleroderma patients have different therapeutic responses to mycophenolate mofetil.
Methods: Self-identified Black and White patients with SSc who had exposure to mycophenolate mofetil (MMF) were selected from the Johns Hopkins Scleroderma Center (JHSC) Research Registry. Patients without at least one PFT and mRSS measurement prior to or within one month of initiation of MMF therapy were excluded. We restricted our study population to patients who presented to our Center within 5 years of disease onset. Our primary outcome measures were absolute change in forced vital capacity (FVC) % predicted and modified Rodnan skin scores (mRSS) over a 5-year period.
Results: 718 patients with SSc who self-identified as either Black (n=152) or White (n=566) were exposed to MMF. Black patients were younger at SSc onset than White patients (mean (SD) 40.1 (13.3) years vs. 47.3 (13.1)), though both groups presented to our Center at a similar disease duration (Table 1). Both groups were comparable in terms of sex and proportion of patients with diffuse cutaneous disease. The most common autoantibody was anti-Scl-70 (46%) for Black patients and anti-RNA polymerase III (46%) for White patients. The majority of subjects in both groups achieved the maximum dose of MMF (3g) for at least 3 months (55% for Black patients, 52% for White patients). In the cohort of patients who had baseline and follow up FVC, the average baseline FVC% predicted was 66.7 (20.0) for Black patients and 83.2 (19.0) for White patients prior to treatment with MMF (Table 2). At 1 year, the modeled absolute change in FVC% predicted for Black patients was +1.7 (1.7) compared to -1.2 (0.7) for White patients (p< 0.001). By year 5, the modeled absolute change in FVC% predicted for Black patients was +1.7 (1.7) compared to -4.9 (0.7) for White patients (p< 0.001). Among patients with dcSSc, the baseline mRSS was 13.3 (11.0) for Black patients and 17.7 (13.7) for White patients (p=0.212). At 1 year, the modeled absolute change in mRSS from baseline was -3.1(1.7) for Black patients and -3.4(1.3) for White patients (p=0.907). By year 5, the modeled absolute change in mRSS for Black patients was -5.4 (1.9) compared to -9.8 (1.3) for White patients (p=0.133).
Conclusion: There is a significant difference in baseline FVC% predicted between Black and White patients, but once treated with MMF, Black patients' FVC% predicted appears to stabilize while White patients have a slow decline. Both groups show a similar reduction in mRSS skin scores with treatment of MMF. Overall, these results suggest that worse pulmonary outcomes for Black patients are not due to reduced therapeutic efficacy but may be due to more severe disease at presentation. Ongoing work is needed to investigate if there is a biologic basis for this differential disease severity and the role of social determinants of health as predictors of worse outcomes in Black versus White SSc patients.
 Table 1. Demographics and clinical characteristics for 718 SSc patients by race
Table 1. Demographics and clinical characteristics for 718 SSc patients by race Table 2. Modeled absolute change in percent FVC and mRSS from start of MMF.
Table 2. Modeled absolute change in percent FVC and mRSS from start of MMF.
Disclosures: L. Smith, None; J. Perin, None; A. Woods, None; F. Wigley, None; L. Hummers, Boehringer-Ingelheim, Corbus Pharmaceuticals, Cumberland Pharmaceuticals, Kadmon Corporation, Medpace, CSL Behring, Mitsubishi Tanabe, Horizon Pharmaceuticals; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation.

