Back
Abstract Session
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: Abstracts: Pediatric Rheumatology – Clinical I: Connective Tissue Disease (0512–0517)
0515: Juvenile Systemic Sclerosis Treatment Practices in an International Cohort and Comparison to Recent SHARE Consensus Guidelines
Saturday, November 12, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 126
- IF
Ivan Foeldvari, MD
Hamburger Zentrum für Kinder- und Jugendrheumatologie
Hamburg, Germany
Presenting Author(s)
Ivan Foeldvari1, Jens Klotsche2, Ozgur Kasapcopur3, Amra Adrovic4, Kathryn Torok5, Maria Teresa Terreri6, Ana Paula Sakamoto7, Brian Feldman8, Jordi Anton9, FLAVIO SZTAJNBOK10, Valda Stanevica11, Simone Appenzeller12, Tadey Avcin13, Sindu Johnson14, Raju Khubchandani15, Mikhail Kostik16, Edoardo Marrani17, Walter Alberto Sifuentes-Giraldo18, Dana Nemcova19, Maria José Santos20, Dieneke Schonenberg-Meinema21, Cristina Battagliotti22, Lillemor Berntson23, Blanca Bica24, Jürgen Brunner24, Rolando Cimaz25, Despina Eleftheriou26, Liora Harel27, Gerd Horneff28, Mahesh Janarthanan29, Tilmann Kallinich30, Thomas Lehman31, Farzana Nuruzzaman32, Anjali Patwardhan33, Vanessa Smith34 and Nicola Helmus35, 1Hamburger Zentrum für Kinder- und Jugendrheumatologie, Hamburg, Germany, 2German Rheumatism Research Center, Berlin, Germany, 3Istanbul University-Cerrahpaşa, Cerrahpaşa Medical School, Istanbul, Turkey, 4Cerrahpaşa Medical School, Istanbul University, Istanbul, Turkey, 5Pediatric Rheumatology, Children's Hospital of UPMC, Pittsburgh, PA, 6Universidad Federal São Paulo, São Paulo, Brazil, 7Federal University of Sao Paulo (UNIFESP), São Paulo, Brazil, 8Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 9Pediatric Rheumatology, Hospital Sant Joan de Déu, Universitat de Barcelona, Barcelona, Spain, 10UFRJ/UERJ, São Paulo, Brazil, 11Children's Clinical University Hospital, Zemgales priekšpilseta, Riga, Latvia, 12Unicamp, Campinas, São Paulo, Brazil, 13University Children's Hospital University Medical Center Ljubljana, Ljubljana, Slovenia, 14University of Toronto, Toronto, ON, Canada, 15SRCC Children's Hospital, Mumbai, India, 16Saint-Petersburg State Pediatric Medical University, Saint Petersburg, Russia, 17University of Florence, Firenze, Italy, 18Hospital Universitario Ramon y Cajal, Madrid, Spain, 19Charles University, Prague, Czech Republic, 20Hospital Garcia de Orta, Almada, Charneca da Caparica, Portugal, 21Emma Children’s Hospital, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands, 22Hospital de Niños Dr Orlando Alassia, Santa Fe, Argentina, 23Dept. of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden, 24UNIVERSIDADE FEDERAL DO RIO DE JANEIRO, Rio de Janeiro, Brazil, 25University of Milano, Milano, Italy, 26Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom, 27Schneider Children's Medical center, Tel Aviv University, Nettnja, Israel, 28Pediatrics, Asklepios Klinik Sankt Augustin GmbH, Sankt Augustin, Germany, 29SRI RAMACHANDRA INSTITUTE OF HIGHER EDUCATION AND RESEARCH, Chennai, India, 30Charité - Universitätsmedizin Berlin, Nuremberg, Germany, 31Hospital for Special Surgery, New York, NY, 32Stony Brook Children's Hospital, East Setauket, NY, 33University of Missouri, Columbia, MO, 34Department of Rheumatology, Ghent University Hospital – Department of Internal Medicine, Ghent University, Belgium – Unit for Molecular Immunology and Inflammation, VIB Inflammation Research Center (IRC), Gent, Belgium, 35Hamburg Centre for Pediatric and Adolescence Rheumatology, Hamburg, Germany
Background/Purpose: Juvenile systemic scleroderma (jSSc) is an orphan disease with a prevalence of 3 in 1,000,000 children. Currently no medications are licensed for the treatment of jSSc. Due to its rarity, only recently have the first management and treatment guidelines been published, the jSSc SHARE (Single Hub and Access point for paediatric Rheumatology in Europe) recommendations, reflecting consensus opinion upon pediatric rheumatologists (1).
Methods: The juvenile systemic sclerosis inceptions cohort (jSScC) is a multinational cohort that prospectively collects clinical data, including medications at baseline and subsequent visits. The jSScC enrollment criteria include age of onset of the first non-Raynaud symptom younger than 16 years and age younger than 18 years at cohort entrance. The frequency of medications (general category and specific medication) was calculated across the cohort at timepoint 0 (enrollment), 12 months and 24 months.
Results: We extracted data from the jSScC of patients who were followed for 12 or 24 months. One-hundred and nine patients were followed at time point 0 (T0) and 12 months (T12), and data was available for 77 of them up at 24 months (T24). The mean age of the patients was 13.2 years at the timepoint 0. Three-quarters were female and 75 % had diffuse subtype. Disease duration at baseline visit was 3.1 years. The medications the patients were on recorded by the physician were captured at T0, T12 and T24 listed in Table 1.
Conclusion: At baseline half of the patients were on corticosteroids. This is more frequent than typical adult SSc practice but coincides with jSSc SHARE treatment recommendations (#1)(1). After 12 months observation in the cohort over 90% of patients received a DMARD therapy. Methotrexate and mycophenolate mofetil were the most commonly prescribed DMARDs, which also reflects the SHARE treatment recommendations (#2 and #3). At 12 months the use of glucocorticoid decreased and the use of bDMARDs increased. In general, biological DMARDs are typically considered in severe or refractory (SHARE recommendation #7), reflecting the lower percentage compared to csDMARDs. Autologous stem cell transplantation was observed in one patient at 12 months, reflecting an option in jSSc with progressive and refractory disease (SHARE recommendation #8). Endothelial receptor antagonists, such as bosentan, were used over time in approximately 20% of the patients, reflecting SHARE recommendation #6 for pulmonary hypertension and/or digital tip ulcers. This is the first evaluation looking at clinical medication practice pattern in jSSc, and its comparison to recently published consensus guidelines.
References:
1. Foeldvari I, Culpo R, Sperotto F, Anton J, Avcin T, Baildam E, et al. Consensus-based recommendations for the management of juvenile systemic sclerosis. Rheumatology (Oxford). 2021;60(4):1651-8.
This project was supported by an unrestricted grant from "Joachim Herz Stiftung"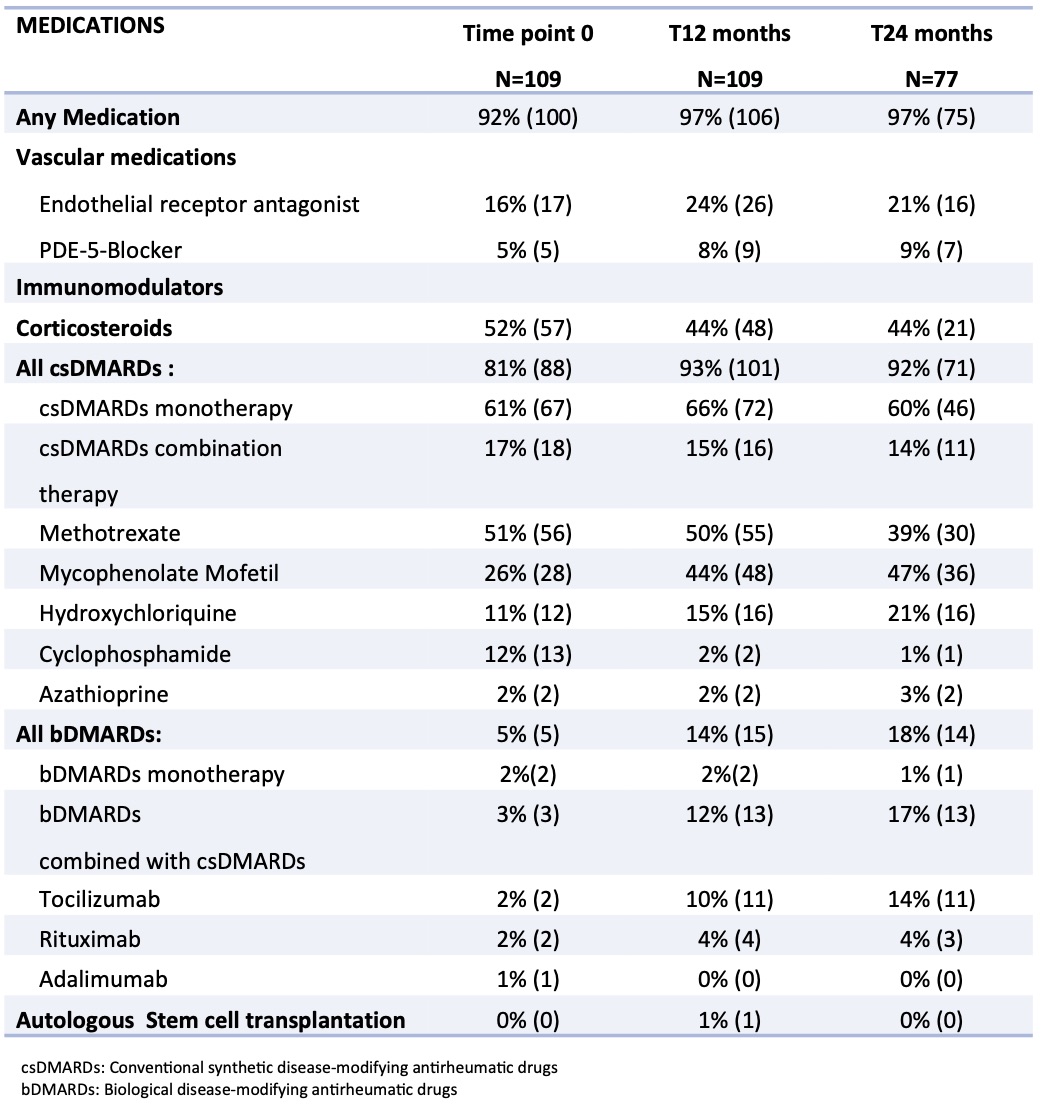
Disclosures: I. Foeldvari, None; J. Klotsche, None; O. Kasapcopur, None; A. Adrovic, None; K. Torok, None; M. Terreri, Roche, Pfizer, UCB, Janssen, Bristol-Myers Squibb(BMS), Eli Lilly, AbbVie/Abbott; A. Sakamoto, None; B. Feldman, Pfizer, AB2-Bio, Janssen; J. Anton, for Sobi, Novimmune, Novartis, Abbvie, Pfizer, GSK, Roche, Amgen, Lilly, BMS, Sanofi, Sobi, Novimmune, Novartis, Pfizer, GSK, Sobi, Novimmune, Novartis, GSK, Pfizer.; F. SZTAJNBOK, None; V. Stanevica, None; S. Appenzeller, None; T. Avcin, None; S. Johnson, None; R. Khubchandani, None; M. Kostik, None; E. Marrani, None; W. Sifuentes-Giraldo, None; D. Nemcova, None; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; D. Schonenberg-Meinema, None; C. Battagliotti, None; L. Berntson, Pfizer; B. Bica, None; J. Brunner, None; R. Cimaz, None; D. Eleftheriou, None; L. Harel, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; M. Janarthanan, None; T. Kallinich, Roche; T. Lehman, None; F. Nuruzzaman, None; A. Patwardhan, None; V. Smith, Boehringer-Ingelheim, Janssen; N. Helmus, None.
Background/Purpose: Juvenile systemic scleroderma (jSSc) is an orphan disease with a prevalence of 3 in 1,000,000 children. Currently no medications are licensed for the treatment of jSSc. Due to its rarity, only recently have the first management and treatment guidelines been published, the jSSc SHARE (Single Hub and Access point for paediatric Rheumatology in Europe) recommendations, reflecting consensus opinion upon pediatric rheumatologists (1).
Methods: The juvenile systemic sclerosis inceptions cohort (jSScC) is a multinational cohort that prospectively collects clinical data, including medications at baseline and subsequent visits. The jSScC enrollment criteria include age of onset of the first non-Raynaud symptom younger than 16 years and age younger than 18 years at cohort entrance. The frequency of medications (general category and specific medication) was calculated across the cohort at timepoint 0 (enrollment), 12 months and 24 months.
Results: We extracted data from the jSScC of patients who were followed for 12 or 24 months. One-hundred and nine patients were followed at time point 0 (T0) and 12 months (T12), and data was available for 77 of them up at 24 months (T24). The mean age of the patients was 13.2 years at the timepoint 0. Three-quarters were female and 75 % had diffuse subtype. Disease duration at baseline visit was 3.1 years. The medications the patients were on recorded by the physician were captured at T0, T12 and T24 listed in Table 1.
Conclusion: At baseline half of the patients were on corticosteroids. This is more frequent than typical adult SSc practice but coincides with jSSc SHARE treatment recommendations (#1)(1). After 12 months observation in the cohort over 90% of patients received a DMARD therapy. Methotrexate and mycophenolate mofetil were the most commonly prescribed DMARDs, which also reflects the SHARE treatment recommendations (#2 and #3). At 12 months the use of glucocorticoid decreased and the use of bDMARDs increased. In general, biological DMARDs are typically considered in severe or refractory (SHARE recommendation #7), reflecting the lower percentage compared to csDMARDs. Autologous stem cell transplantation was observed in one patient at 12 months, reflecting an option in jSSc with progressive and refractory disease (SHARE recommendation #8). Endothelial receptor antagonists, such as bosentan, were used over time in approximately 20% of the patients, reflecting SHARE recommendation #6 for pulmonary hypertension and/or digital tip ulcers. This is the first evaluation looking at clinical medication practice pattern in jSSc, and its comparison to recently published consensus guidelines.
References:
1. Foeldvari I, Culpo R, Sperotto F, Anton J, Avcin T, Baildam E, et al. Consensus-based recommendations for the management of juvenile systemic sclerosis. Rheumatology (Oxford). 2021;60(4):1651-8.
This project was supported by an unrestricted grant from "Joachim Herz Stiftung"

Disclosures: I. Foeldvari, None; J. Klotsche, None; O. Kasapcopur, None; A. Adrovic, None; K. Torok, None; M. Terreri, Roche, Pfizer, UCB, Janssen, Bristol-Myers Squibb(BMS), Eli Lilly, AbbVie/Abbott; A. Sakamoto, None; B. Feldman, Pfizer, AB2-Bio, Janssen; J. Anton, for Sobi, Novimmune, Novartis, Abbvie, Pfizer, GSK, Roche, Amgen, Lilly, BMS, Sanofi, Sobi, Novimmune, Novartis, Pfizer, GSK, Sobi, Novimmune, Novartis, GSK, Pfizer.; F. SZTAJNBOK, None; V. Stanevica, None; S. Appenzeller, None; T. Avcin, None; S. Johnson, None; R. Khubchandani, None; M. Kostik, None; E. Marrani, None; W. Sifuentes-Giraldo, None; D. Nemcova, None; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; D. Schonenberg-Meinema, None; C. Battagliotti, None; L. Berntson, Pfizer; B. Bica, None; J. Brunner, None; R. Cimaz, None; D. Eleftheriou, None; L. Harel, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; M. Janarthanan, None; T. Kallinich, Roche; T. Lehman, None; F. Nuruzzaman, None; A. Patwardhan, None; V. Smith, Boehringer-Ingelheim, Janssen; N. Helmus, None.

