Back
Abstract Session
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics (0518–0523)
0523: The Development of the Ranked Composite Important Difference in Diffuse Cutaneous Systemic Sclerosis; A Clinical and Patient Meaningful Anchor to the ACR-Composite Response Index in SSc
Saturday, November 12, 2022
4:15 PM – 4:25 PM Eastern Time
Location: Room 121

Francesco Del Galdo, MD, PhD
University of Leeds
Leeds, United Kingdom
Presenting Author(s)
Lesley-Anne Bissell1, Daniel Furst2, sindhu johnson3, Paul hansen4, Esmeralda Recalde5, Dinesh Khanna6 and Francesco Del Galdo7, 1University of Leeds, Leeds, United Kingdom, 2University of California Los Angeles, Los Angeles, CA, 3Rheumatology, Department of Medicine, Schroeder Arthritis Institute, Toronto Western Hospital, Mount Sinai Hospital; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada, 4University of Otago, Dunedin, New Zealand, 5World Scleroderma Foundation, Florence, Italy, 6Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI, 7Leeds Institute of Rheumatic and Musculoskeletal Medicine, Faculty of Medicine and Health and NIHR Biomedical Research Centre, University of Leeds, Leeds, United Kingdom
Background/Purpose: The ACR Composite Response Index in Systemic Sclerosis ( ACR-CRISS) is one of the first composite outcome measures in diffuse cutaneous Systemic sclerosis (dcSSc- Khanna D, et al. A&R 2016) . It relies on validated clinical domains selected through a data driven methodology, however only provides a probability of response and is unable to differentiate between patients who do not improve and patients who worsen.
Here we aimed to improve the clinical interpretation of the ACR-CRISS by creating a continuous ranked score of clinically and patient meaningful changes of its individual measures.
Methods: Following OmerACT guidelines for outcome measurement development, relevant stakeholders were identified from 5 continents, including 100 physicians with proven experience in managing patients with dcSSc and 100 patients with dcSSc who have participated to at least one clinical trial. An adaptive survey was designed utilising 1000Minds conjoint analysis software. Patients and doctors were asked to choose which of two hypothetical patients had a better or worse outcome according to Minimally Clinical Important Differences (MCID) in two domains among FVC, HAQ-DI and mRSS, and the presence of organ failure. Pairwise comparisons of patient and doctor choices were analysed utilising PAPRIKA methodology (Hansen P et al Multi-Criteria Decis. Anal 2008) to rank and weight the MCIDs against each other. Working jointly with patient and public involvement utilising a 'think aloud' approach, a video tutorial was produced explaining the objectives and process of the adaptive survey to the survey participants.
Results: 80 rheumatologists and 80 patients with Scleroderma accepted to complete the survey which ran from June 2020 to January 2021. Discrete choices conjoint analysis defined ranks and relative weights of the 4 domains in the improving and worsening outcomes. A continuous composite ranked score reflecting the relative weighting of the individual outcome measures (Ranked Composite Important Difference, RCID) was developed accordingly (Table 1). The score ranges from -1 (worst possible outcome) to 1 (best possible outcome), with patients that experience no organ failure and do not meet any MCID in any of the 3 domains scoring 0.
Conclusion: This collaborative process using novel robust methodology and involving both rheumatologists and patients has created a clinically and patient meaningful composite score that can be used as anchor to the ACR-CRISS, or other clinical outcomes. Performance against the ACR-CRISS, and revised CRISS in Randomised Controlled Trials will determine the clinical value of the RCID.
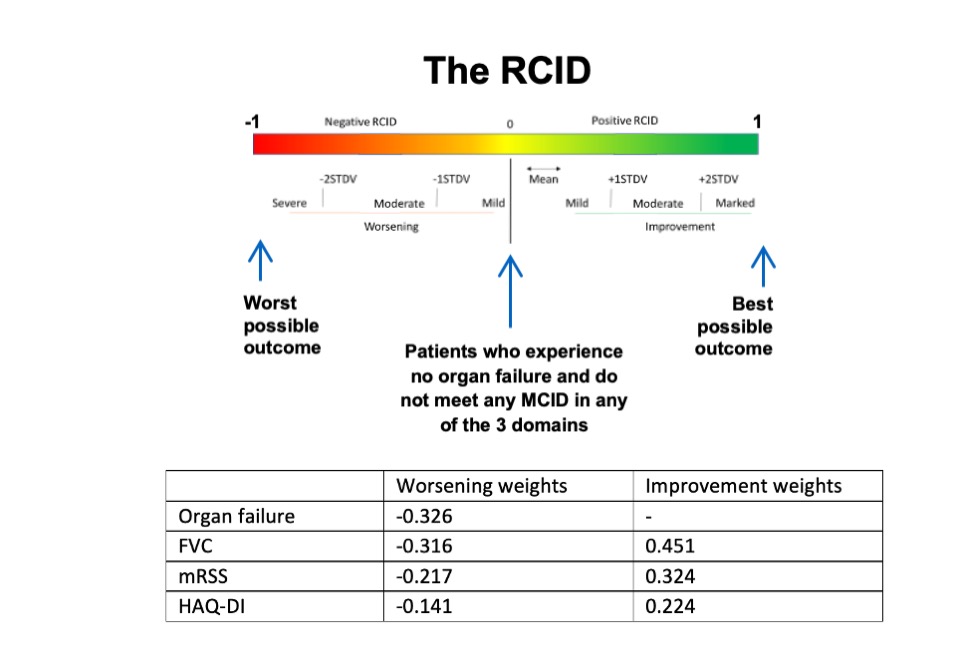 The relative weights of each of the core set measures within the better and worse outcome models, expressed as a score from -1 to 1 and the brackets for RCID scoring.
The relative weights of each of the core set measures within the better and worse outcome models, expressed as a score from -1 to 1 and the brackets for RCID scoring.
Disclosures: L. Bissell, None; D. Furst, AbbVie/Abbott, Pfizer, CORBUS, GALAPAGOS, GlaxoSmithKlein(GSK), NIH, Novartis, HORIZON, PROMETHEUS, SANOFI, Roche, Genentech, GALDERMA, CME, Bristol-Myers Squibb(BMS), Amgen; s. johnson, None; P. hansen, None; E. Recalde, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; F. Del Galdo, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Mitsubishi-Tanabe, Capella biosciences, Chemomab LTD, Kymab.
Background/Purpose: The ACR Composite Response Index in Systemic Sclerosis ( ACR-CRISS) is one of the first composite outcome measures in diffuse cutaneous Systemic sclerosis (dcSSc- Khanna D, et al. A&R 2016) . It relies on validated clinical domains selected through a data driven methodology, however only provides a probability of response and is unable to differentiate between patients who do not improve and patients who worsen.
Here we aimed to improve the clinical interpretation of the ACR-CRISS by creating a continuous ranked score of clinically and patient meaningful changes of its individual measures.
Methods: Following OmerACT guidelines for outcome measurement development, relevant stakeholders were identified from 5 continents, including 100 physicians with proven experience in managing patients with dcSSc and 100 patients with dcSSc who have participated to at least one clinical trial. An adaptive survey was designed utilising 1000Minds conjoint analysis software. Patients and doctors were asked to choose which of two hypothetical patients had a better or worse outcome according to Minimally Clinical Important Differences (MCID) in two domains among FVC, HAQ-DI and mRSS, and the presence of organ failure. Pairwise comparisons of patient and doctor choices were analysed utilising PAPRIKA methodology (Hansen P et al Multi-Criteria Decis. Anal 2008) to rank and weight the MCIDs against each other. Working jointly with patient and public involvement utilising a 'think aloud' approach, a video tutorial was produced explaining the objectives and process of the adaptive survey to the survey participants.
Results: 80 rheumatologists and 80 patients with Scleroderma accepted to complete the survey which ran from June 2020 to January 2021. Discrete choices conjoint analysis defined ranks and relative weights of the 4 domains in the improving and worsening outcomes. A continuous composite ranked score reflecting the relative weighting of the individual outcome measures (Ranked Composite Important Difference, RCID) was developed accordingly (Table 1). The score ranges from -1 (worst possible outcome) to 1 (best possible outcome), with patients that experience no organ failure and do not meet any MCID in any of the 3 domains scoring 0.
Conclusion: This collaborative process using novel robust methodology and involving both rheumatologists and patients has created a clinically and patient meaningful composite score that can be used as anchor to the ACR-CRISS, or other clinical outcomes. Performance against the ACR-CRISS, and revised CRISS in Randomised Controlled Trials will determine the clinical value of the RCID.
 The relative weights of each of the core set measures within the better and worse outcome models, expressed as a score from -1 to 1 and the brackets for RCID scoring.
The relative weights of each of the core set measures within the better and worse outcome models, expressed as a score from -1 to 1 and the brackets for RCID scoring.Disclosures: L. Bissell, None; D. Furst, AbbVie/Abbott, Pfizer, CORBUS, GALAPAGOS, GlaxoSmithKlein(GSK), NIH, Novartis, HORIZON, PROMETHEUS, SANOFI, Roche, Genentech, GALDERMA, CME, Bristol-Myers Squibb(BMS), Amgen; s. johnson, None; P. hansen, None; E. Recalde, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; F. Del Galdo, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Mitsubishi-Tanabe, Capella biosciences, Chemomab LTD, Kymab.

