Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Diagnosis, Manifestations, and Outcomes I: Pre- and Early Disease (0530–0535)
0530: Abatacept Significantly Reduces Subclinical Inflammation During Treatment (6 Months), This Persists After Discontinuation (12 Months), Resulting in a Delay in the Clinical Development of RA in Patients at Risk of RA (The ARIAA Study)
Saturday, November 12, 2022
4:30 PM – 4:40 PM Eastern Time
Location: Ballroom AB
.png)
Juergen Rech, MD
University Clinic Erlangen
Erlangen, Germany
Presenting Author(s)
Juergen Rech1, Arnd Kleyer2, Mikkel Østergaard3, Melanie Hagen2, Larissa Valor Mendez2, Koray Tascilar2, Gerhard Kroenke2, Verena Schönau2, David Simon2, stefan Kleinert4, Xenofon Baraliakos5, Juergen Braun6, Axel Hueber7, Martin Fleck8, Andrea Rubbert-Roth9, Frank Behrens10, Martin Feuchtenberger11, M. Zaenker12, Reinhard Voll13, Cornelia Glaser14, Mária Filková15, Eugen Feist16, Gerd Burmester17, Kirsten Karberg18, Johannes Strunk19, Juan Canete20, Ladislav Šenolt15, Esperanza Naredo21 and Georg Schett22, 1University Clinic Erlangen, Erlangen, Germany, 2Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-UniversityErlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany, 3Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 4Praxisgemeinschaft Rheumatologie - Nephrologie (PGRN), Erlangen, Germany, 5Ruhr University Bochum, Department of Rheumatology, Bochum, Germany; Rheumazentrum Ruhrgebiet, Rheumazentrum Ruhrgebiet, Herne, Germany, 6Rheumazentrum Ruhrgebiet, Herne, Germany, 7Klinikum Nuernberg Nord, Abteilung fuer Rheumatologie, Nuernberg, Germany, 8Asklepios Klinikum Bad Abbach, Klinik und Poliklinik für Rheumatologie/ Klin. Immunologie, Bad Abbach, Germany, 9Division of Rheumatology, Cantonal Clinic St Gallen, St.Gallen, Switzerland, 10Division of Rheumatology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany; Fraunhofer Institute for Translational Medicine & Pharmacology ITMP, Frankfurt, 11MED | BAYERN OST GmbH, Fachbereich Rheumatologie, Burghausen, Germany, 12Immanuel Klinikum Bernau , Herzzentrum Brandenburg, Abteilung Innere Medizin, Bernau, Germany, 13Universitätsklinikum Freiburg, Klinik für Rheumatologie und Klinische Immunologie, Freiburg im Breisgau, Germany, 14Universitätsklinikum Freiburg, Klinik für Rheumatologie und Klinische Immunologie, Freiburg, Germany, 15Institute of Rheumatology and Department of Rheumatology, First Faculty of Medicine, Charles University, Prague, Czech Republic, 16Helios Clinic Vogelsang-Gommern, cooperation partner of the Otto von Guericke University Magdeburg, Magdeburg, Germany, 17Charité University Medicine Berlin, Berlin, Germany, 18Praxis für Rheumatologie und Innere Medizin, Rheumatologie, Berlin, Germany, 19Krankenhaus Porz am Rhein GmbH, Rheumaklinik, Köln (Porz), Germany, Koeln, Germany, 20Unidad de Artritis Hospital Clinic, Barcelona, Spain, 21Hospital General Universitario Gregorio Marañón and Complutense University, Madrid, Spain, 22Universitätsklinikum Erlangen, Erlangen, Germany
Background/Purpose: Development of the clinical phase of rheumatoid arthritis (RA) is preceded by an autoimmune phase, characterized by the presence of anti-citrullinated protein antibodies (ACPA), that gradually translates into clinical disease. Individuals with ACPA, but no clinical arthritis, that show signs of subclinical joint inflammation in the imaging are at particularly high risk to develop RA within a short time. We have previously shown that a 6-months treatment with abatacept not only inhibits the onset of RA but also reduces MRI signs of inflammation in such ACPA-positive high-risk individuals. However, it is currently unknown whether such time-limited intervention leads to sustained suppression of inflammation in the joints.
To test whether time-limited treatment of abatacept has a sustained effect on MRI-based joint inflammation one year after the cessation of treatment
Methods: ARIAA is an international, randomized double-blinded placebo-controlled multi-center study in RA-at risk individuals, being ACPA positive and showing MRI signs of inflammation. The study was composed of a 6 months treatment phase with either abatacept s.c. 125 mg weekly or placebo and a 12 months observation phase with no treatment. Primary endpoint was the improvement of MRI inflammation after 6 months, secondary endpoints were the progression to RA after 6 and 18 months. The primary analysis was done on the ITT population and missing values were classified as treatment failures.
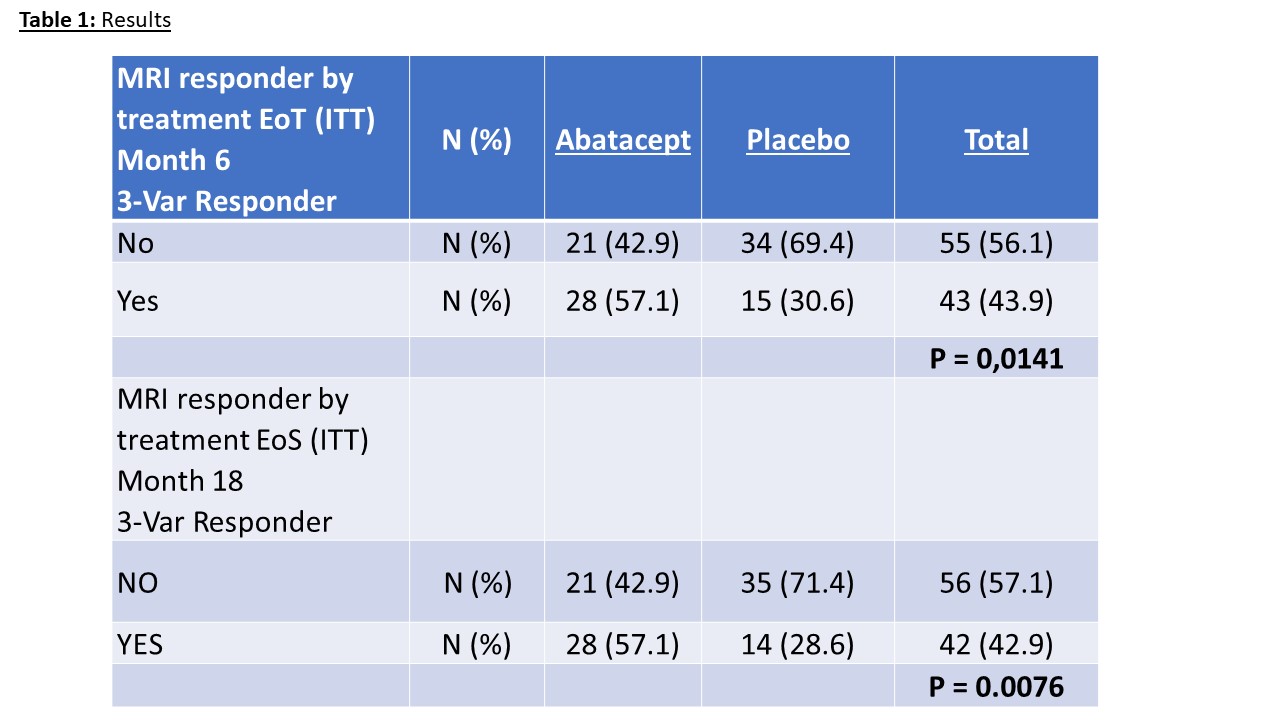
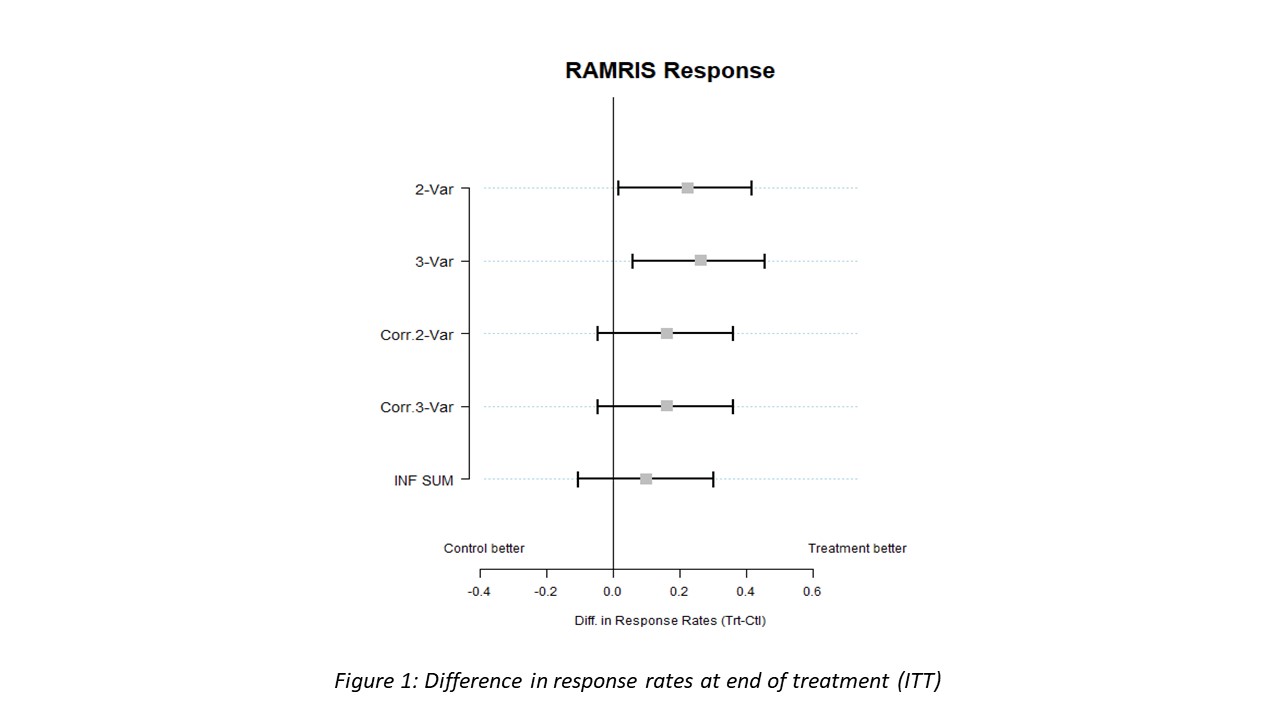
Results: Between November 2014 and December 2019 139 RA-at risk individuals were included into ARIAA by 14 study sites (11 in Germany, 1 in the Czech Republic and 2 in Spain). Of them, 100 patients were randomized to receive either abatacept or placebo. Two patients were excluded and 98 patients could be evaluated for efficacy and safety. As reported previously the primary endpoint (improvement in RAMRIS synovitis (S), tenosynovitis score (TS) or osteitis score (O) at 6 month) was met. Also, key secondary endpoints (progression to RA after 6 and 18 months) were met in the ARIAA study. Most importantly, even after 18 months, after a 12-month cessation of abatacept, progression to RA was lower in the abatacept group (35%) than in the placebo group (57%; p=0.0076). With respect to MRI findings at 18 months, the analysis showed that 28 patients (57.1%) in the abatacept group were MRI responders while only 14 patients (28.6.9%) in the placebo group had an improvement in synovitis, or tenosynovitis or osteitis (p=0.0076).
Conclusion: These data show that the effects of abatacept on MRI joint inflammation observed after 6 months of treatment persist after discontinuation of the drug over a period of 12-months. These data further support the value of early disease intervention in the pre-phase of arthritis and indicate that early time-limited treatment has sustained effects on disease outcome.
Disclosures: J. Rech, Novartis, SOBI, AbbVie/Abbott, Biogen, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Merck/MSD, Mylan, Roche, Sanofi, UCB; A. Kleyer, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Hagen, None; L. Valor Mendez, None; K. Tascilar, Gılead, AbbVie/Abbott, UCB, Eli Lilly; G. Kroenke, None; V. Schönau, None; D. Simon, None; s. Kleinert, None; X. Baraliakos, None; J. Braun, None; A. Hueber, None; M. Fleck, None; A. Rubbert-Roth, None; F. Behrens, None; M. Feuchtenberger, None; M. Zaenker, None; R. Voll, None; C. Glaser, None; M. Filková, None; E. Feist, AbbVie, Lilly, Galapagos, Novartis, Pfizer, Roche, SOBI, BMS, MSD; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; K. Karberg, None; J. Strunk, None; J. Canete, None; L. Šenolt, None; E. Naredo, AbbVie, Roche, Bristol-Myers Squibb(BMS), Pfizer, UCB, Eli Lilly, Novartis, Janssen, Celgene, GmbH; G. Schett, None.
Background/Purpose: Development of the clinical phase of rheumatoid arthritis (RA) is preceded by an autoimmune phase, characterized by the presence of anti-citrullinated protein antibodies (ACPA), that gradually translates into clinical disease. Individuals with ACPA, but no clinical arthritis, that show signs of subclinical joint inflammation in the imaging are at particularly high risk to develop RA within a short time. We have previously shown that a 6-months treatment with abatacept not only inhibits the onset of RA but also reduces MRI signs of inflammation in such ACPA-positive high-risk individuals. However, it is currently unknown whether such time-limited intervention leads to sustained suppression of inflammation in the joints.
To test whether time-limited treatment of abatacept has a sustained effect on MRI-based joint inflammation one year after the cessation of treatment
Methods: ARIAA is an international, randomized double-blinded placebo-controlled multi-center study in RA-at risk individuals, being ACPA positive and showing MRI signs of inflammation. The study was composed of a 6 months treatment phase with either abatacept s.c. 125 mg weekly or placebo and a 12 months observation phase with no treatment. Primary endpoint was the improvement of MRI inflammation after 6 months, secondary endpoints were the progression to RA after 6 and 18 months. The primary analysis was done on the ITT population and missing values were classified as treatment failures.
Results: Between November 2014 and December 2019 139 RA-at risk individuals were included into ARIAA by 14 study sites (11 in Germany, 1 in the Czech Republic and 2 in Spain). Of them, 100 patients were randomized to receive either abatacept or placebo. Two patients were excluded and 98 patients could be evaluated for efficacy and safety. As reported previously the primary endpoint (improvement in RAMRIS synovitis (S), tenosynovitis score (TS) or osteitis score (O) at 6 month) was met. Also, key secondary endpoints (progression to RA after 6 and 18 months) were met in the ARIAA study. Most importantly, even after 18 months, after a 12-month cessation of abatacept, progression to RA was lower in the abatacept group (35%) than in the placebo group (57%; p=0.0076). With respect to MRI findings at 18 months, the analysis showed that 28 patients (57.1%) in the abatacept group were MRI responders while only 14 patients (28.6.9%) in the placebo group had an improvement in synovitis, or tenosynovitis or osteitis (p=0.0076).
Conclusion: These data show that the effects of abatacept on MRI joint inflammation observed after 6 months of treatment persist after discontinuation of the drug over a period of 12-months. These data further support the value of early disease intervention in the pre-phase of arthritis and indicate that early time-limited treatment has sustained effects on disease outcome.


Disclosures: J. Rech, Novartis, SOBI, AbbVie/Abbott, Biogen, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Merck/MSD, Mylan, Roche, Sanofi, UCB; A. Kleyer, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Hagen, None; L. Valor Mendez, None; K. Tascilar, Gılead, AbbVie/Abbott, UCB, Eli Lilly; G. Kroenke, None; V. Schönau, None; D. Simon, None; s. Kleinert, None; X. Baraliakos, None; J. Braun, None; A. Hueber, None; M. Fleck, None; A. Rubbert-Roth, None; F. Behrens, None; M. Feuchtenberger, None; M. Zaenker, None; R. Voll, None; C. Glaser, None; M. Filková, None; E. Feist, AbbVie, Lilly, Galapagos, Novartis, Pfizer, Roche, SOBI, BMS, MSD; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; K. Karberg, None; J. Strunk, None; J. Canete, None; L. Šenolt, None; E. Naredo, AbbVie, Roche, Bristol-Myers Squibb(BMS), Pfizer, UCB, Eli Lilly, Novartis, Janssen, Celgene, GmbH; G. Schett, None.

