Back
Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including PsA – Treatment I: Axial Spondyloarthritis (0542–0547)
0546: COmparison of the Effect of Treatment with NSAIDs Added to Anti-TNF Therapy versus Anti-TNF Therapy Alone on Progression of StrUctural Damage in the Spine over Two Years in Patients with Ankylosing Spondylitis (CONSUL): An Open-Label, Randomized Controlled, Multicenter Trial
Saturday, November 12, 2022
5:30 PM – 5:40 PM Eastern Time
Location: Room 204
- FP
Fabian Proft, MD
Charité Universityhospital Berlin
Berlin, Germany
Presenting Author(s)
Fabian Proft1, Bukrhard Muche2, valeria Rios-Rodriguez3, Murat Torgutalp4, Mikhail Protopopov5, Joachim Listing6, Maryna Verba7, Jan Brandt-Juergens8, Uta Kiltz9, Maren Sieburg10, Swen Jacki11, Joachim Sieper4 and Denis Poddubnyy1, 1Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Charité University Hospital, CCM, Berlin, Germany, 3Charité-Universitätsmedizin Berlin, Berlin, Germany, 4Charité - Universitätsmedizin, Berlin, Berlin, Germany, 5Charité Universitätsmedizin Berlin, Berlin, Germany, 6German Rheumatism Research Centre, Epidemiology Unit, Berlin, Germany, 7Charité – Universitätsmedizin Berlin, Department of Gastroenterology, Infectiology and Rheumatology (including Nutrition Medicine), Berlin, Germany, 8Rheumatologische Schwerpunktpraxis, Berlin, Germany, 9Rheumazentrum Ruhrgebiet, Herne, Germany, 10Rheumatologische Facharztpraxis, Magdeburg, Germany, 11Rheumatologische Schwerpunktpraxis, Tübingen, Germany
Background/Purpose: There is some evidence that NSAIDs, in particular celecoxib (CEL), might possess not only symptomatic efficacy but also disease-modifying properties in ankylosing spondylitis (AS), retarding progression of structural damage in the spine if taken continuously. For biological disease-modifying antirheumatic drugs (bDMARDs), retardation of structural damage progression has also been demonstrated, but at least 4 years of treatment seem to be necessary (at least for tumour necrosis factor inhibitors – TNFi) to see such an effect. Therefore, a combination of an NSAID with a TNFi might bring additional benefits in terms of retardation of structural damage progression especially in high-risk patients.
The aim of this RCT was to evaluate the impact of treatment with the COX-II-selective NSAID (CEL) when added to a TNFi (golimumab - GOL) compared with TNFi (GOL) alone on progression of structural damage in the spine over 2 years in patients with r-axSpA.
Methods: Eligible patients had r-axSpA and high disease activity (BASDAI ≥4), NSAID failure and risk factors for radiographic spinal progression: C-reactive protein >5 mg/l and/or ≥1 syndesmophyte(s). The trial consisted of two phases: a 12-week run-in phase, in which all included patients received treatment with GOL 50 mg every 4 weeks sc, followed by a 96-week controlled treatment period, in which patients who achieved a BASDAI improvement of ≥2 points were randomly assigned to GOL + CEL 200 mg bid or GOL alone arms. The primary endpoint was radiographic spinal progression as assessed by the change in the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) after 108 weeks in the intent-to-treat population, read by 3 independent readers blinded for the treatment arm and the time-point.
Results: Of the 157 screened patients, 81.5% (n=128) were enrolled into the run-in phase. 109 patients fulfilled the BASDAI response criterion at w12 and were randomized 1:1 (54 vs. 55) to GOL+CEL or GOL alone; 97 (45 vs. 52) patients completed the study at w108. Clinical characteristics of the randomized patients are shown in Tab. 1. The mSASSS change after w108 was 1.1 (95%CI 0.2; 2.0) vs. 1.7 (95%CI 0.8; 2.6) in the GOL+CEL vs. GOL alone groups, respectively, p=0.79. Fig. 1 shows the cumulative probability of the mSASSS change in both treatment arms. New syndesmophytes in the opinion of three readers occurred in 11% vs. 25% of the patients in the GOL+CEL vs. GOL alone groups, respectively, p=0.12. During the study, a total of 14 serious adverse events (SAE) were reported (7 in the GOL+CEL group, 5 in the GOL alone group and 2 during the run-in phase).
Conclusion: In this study, a combined therapy with GOL+CEL did not show significant superiority over GOL monotherapy in retarding radiographic spinal progression over two years in r-axSpA patients. However, the observed numerical reduction in radiographic spinal progression associated with the combined treatment might be, clinically relevant for patients at high risk for progression.
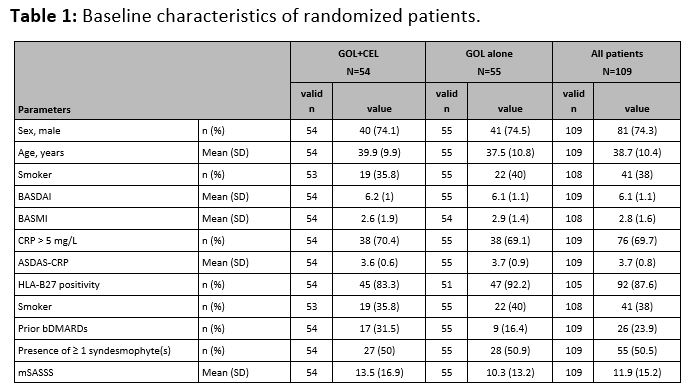 Table 1: Baseline characteristics of randomized patients.
Table 1: Baseline characteristics of randomized patients.
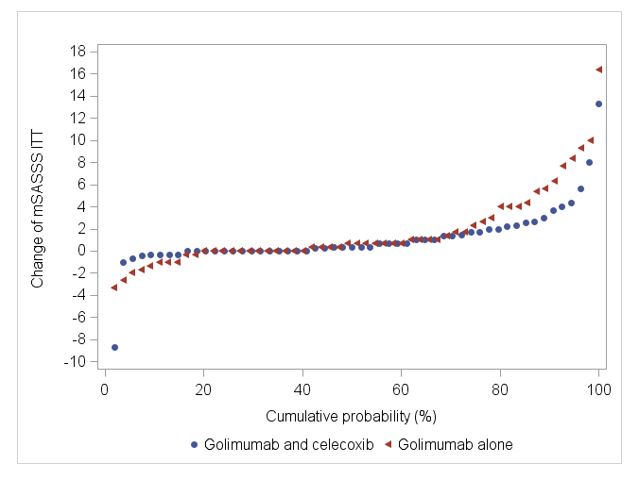 Figure 1: Cumulative probability plot of mSASSS progression over 108 weeks of treatment.
Figure 1: Cumulative probability plot of mSASSS progression over 108 weeks of treatment.
Disclosures: F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; B. Muche, Amgen, Gilead, Galapagos, UCB and Stadapharm, Kyowa Kirin; v. Rios-Rodriguez, Falk, AbbVie/Abbott; M. Torgutalp, UCB, AbbVie/Abbott; M. Protopopov, None; J. Listing, None; M. Verba, None; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; M. Sieburg, None; S. Jacki, None; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.
Background/Purpose: There is some evidence that NSAIDs, in particular celecoxib (CEL), might possess not only symptomatic efficacy but also disease-modifying properties in ankylosing spondylitis (AS), retarding progression of structural damage in the spine if taken continuously. For biological disease-modifying antirheumatic drugs (bDMARDs), retardation of structural damage progression has also been demonstrated, but at least 4 years of treatment seem to be necessary (at least for tumour necrosis factor inhibitors – TNFi) to see such an effect. Therefore, a combination of an NSAID with a TNFi might bring additional benefits in terms of retardation of structural damage progression especially in high-risk patients.
The aim of this RCT was to evaluate the impact of treatment with the COX-II-selective NSAID (CEL) when added to a TNFi (golimumab - GOL) compared with TNFi (GOL) alone on progression of structural damage in the spine over 2 years in patients with r-axSpA.
Methods: Eligible patients had r-axSpA and high disease activity (BASDAI ≥4), NSAID failure and risk factors for radiographic spinal progression: C-reactive protein >5 mg/l and/or ≥1 syndesmophyte(s). The trial consisted of two phases: a 12-week run-in phase, in which all included patients received treatment with GOL 50 mg every 4 weeks sc, followed by a 96-week controlled treatment period, in which patients who achieved a BASDAI improvement of ≥2 points were randomly assigned to GOL + CEL 200 mg bid or GOL alone arms. The primary endpoint was radiographic spinal progression as assessed by the change in the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) after 108 weeks in the intent-to-treat population, read by 3 independent readers blinded for the treatment arm and the time-point.
Results: Of the 157 screened patients, 81.5% (n=128) were enrolled into the run-in phase. 109 patients fulfilled the BASDAI response criterion at w12 and were randomized 1:1 (54 vs. 55) to GOL+CEL or GOL alone; 97 (45 vs. 52) patients completed the study at w108. Clinical characteristics of the randomized patients are shown in Tab. 1. The mSASSS change after w108 was 1.1 (95%CI 0.2; 2.0) vs. 1.7 (95%CI 0.8; 2.6) in the GOL+CEL vs. GOL alone groups, respectively, p=0.79. Fig. 1 shows the cumulative probability of the mSASSS change in both treatment arms. New syndesmophytes in the opinion of three readers occurred in 11% vs. 25% of the patients in the GOL+CEL vs. GOL alone groups, respectively, p=0.12. During the study, a total of 14 serious adverse events (SAE) were reported (7 in the GOL+CEL group, 5 in the GOL alone group and 2 during the run-in phase).
Conclusion: In this study, a combined therapy with GOL+CEL did not show significant superiority over GOL monotherapy in retarding radiographic spinal progression over two years in r-axSpA patients. However, the observed numerical reduction in radiographic spinal progression associated with the combined treatment might be, clinically relevant for patients at high risk for progression.
 Table 1: Baseline characteristics of randomized patients.
Table 1: Baseline characteristics of randomized patients. Figure 1: Cumulative probability plot of mSASSS progression over 108 weeks of treatment.
Figure 1: Cumulative probability plot of mSASSS progression over 108 weeks of treatment.Disclosures: F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; B. Muche, Amgen, Gilead, Galapagos, UCB and Stadapharm, Kyowa Kirin; v. Rios-Rodriguez, Falk, AbbVie/Abbott; M. Torgutalp, UCB, AbbVie/Abbott; M. Protopopov, None; J. Listing, None; M. Verba, None; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; M. Sieburg, None; S. Jacki, None; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.

