Back
Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes I: Renal Aspects (0536–0541)
0540: Evaluation of Traditional Laboratory Markers as Predictors of Renal Flares: A Post-hoc Analysis of Four Phase III Clinical Trials of SLE
Saturday, November 12, 2022
5:30 PM – 5:40 PM Eastern Time
Location: Room 122
- AG
Alvaro Gomez, MD
Karolinska Institutet
Stockholm, Sweden
Presenting Author(s)
Sandra Jägerback1, Alvaro Gomez1 and Ioannis Parodis2, 1Division of Rheumatology, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden, 2Karolinska Institutet, Stockholm, Sweden
Background/Purpose: In patients with systemic lupus erythematosus (SLE), renal involvement is associated with high morbidity, and renal flare is a major contributing factor to poor long-term prognosis. Lupus nephritis (LN) still leads to end-stage kidney disease in approximately 10–20 % of SLE patients. Identification of patients with susceptibility to develop a renal flare despite immunosuppressant therapy is needed toward individualised treatment optimisation, which was the scope of the present investigation.
Methods: In this study, we used pooled data from four phase III randomised controlled trials of belimumab in patients with SLE, i.e. BLISS-52 (NCT01597622; N=865), BLISS-76 (NCT00410384; N=819), BLISS Northeast Asia (NCT1597622; N=677), and BLISS-SC (NCT01484496; N=836). SLE patients with moderate to severe disease activity were recruited while active severe LN was an exclusion criterion. Participants were on non-biologic standard therapy, and were assigned to IV belimumab (1 mg/kg or 10 mg/kg every fourth week), SC belimumab 200 mg weekly, or placebo. The outcome of this investigation was development of renal flares, defined as a reproducible (i) increase in proteinuria to >1 g/day if the baseline value was < 0.2 g/d, >2 g/day if the baseline value was 0.2–1.0 g/d, or >2 times the baseline value if the baseline value was >1g/d, (ii) increase in serum creatinine ≥20% or 0.3 mg/dL, accompanied by proteinuria, haematuria or red blood cell (RBC) casts, or (iii) new haematuria of glomerular origin, accompanied by proteinuria or RBC casts. Cox proportional hazards regression models were employed. We next adjusted the models for age, sex, ethnicity, BMI, organ damage, baseline SLE disease activity, previous renal involvement, baseline prednisone equivalent dose, and use of immunosuppressants and belimumab.
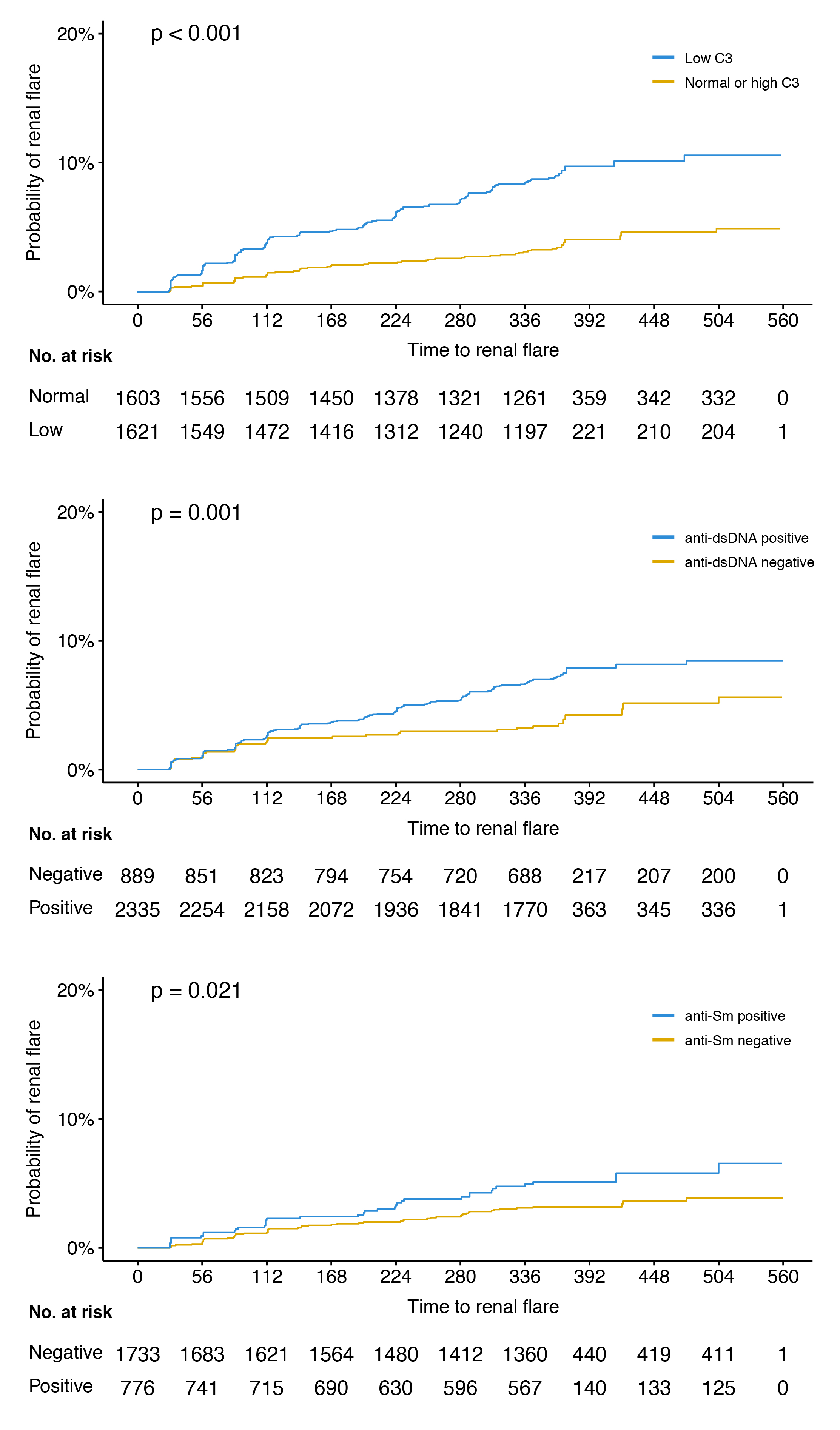
Results: The mean age of the patients was 36.7 years, 94% were women, and 192 developed a renal flare after a median of 141 days. The proportion of patients with a history of renal involvement was 54.6%. In univariable Cox regression analysis, low C3 levels at baseline were associated with an increased risk to develop a renal flare (hazard ratio, HR: 2.6; 95% confidence interval, CI: 1.9–3.5; p< 0.001; figure), as did anti-dsDNA positivity (HR: 1.8; 95% CI: 1.3–2.6; p=0.001), anti-Sm positivity (HR: 1.6; 95% CI: 1.1–2.5; p=0.022), plasma albumin levels (HR: 0.9; 95% CI: 0.8–0.9; p< 0.001), and proteinuria (HR: 1.5; 95% CI: 1.4–1.6; p< 0.001). Low C4 levels were not associated with renal flare development. In adjusted analysis, the association with low C3 levels, plasma albumin and proteinuria remained significant.
Conclusion: Low C3, plasma albumin and proteinuria levels at baseline were associated with renal flare development in a clinical trial setting encompassing patients with active SLE yet no severe active LN, as were anti-dsDNA and anti-Sm positivity yet only in unadjusted models. While C3 and anti-dsDNA are markers of known importance for surveillance of renal SLE, anti-Sm and plasma albumin are not established markers of renal flare; investigation of potential complemental predictive properties across these traditional biomarkers has merit.
Disclosures: S. Jägerback, AstraZeneca; A. Gomez, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.
Background/Purpose: In patients with systemic lupus erythematosus (SLE), renal involvement is associated with high morbidity, and renal flare is a major contributing factor to poor long-term prognosis. Lupus nephritis (LN) still leads to end-stage kidney disease in approximately 10–20 % of SLE patients. Identification of patients with susceptibility to develop a renal flare despite immunosuppressant therapy is needed toward individualised treatment optimisation, which was the scope of the present investigation.
Methods: In this study, we used pooled data from four phase III randomised controlled trials of belimumab in patients with SLE, i.e. BLISS-52 (NCT01597622; N=865), BLISS-76 (NCT00410384; N=819), BLISS Northeast Asia (NCT1597622; N=677), and BLISS-SC (NCT01484496; N=836). SLE patients with moderate to severe disease activity were recruited while active severe LN was an exclusion criterion. Participants were on non-biologic standard therapy, and were assigned to IV belimumab (1 mg/kg or 10 mg/kg every fourth week), SC belimumab 200 mg weekly, or placebo. The outcome of this investigation was development of renal flares, defined as a reproducible (i) increase in proteinuria to >1 g/day if the baseline value was < 0.2 g/d, >2 g/day if the baseline value was 0.2–1.0 g/d, or >2 times the baseline value if the baseline value was >1g/d, (ii) increase in serum creatinine ≥20% or 0.3 mg/dL, accompanied by proteinuria, haematuria or red blood cell (RBC) casts, or (iii) new haematuria of glomerular origin, accompanied by proteinuria or RBC casts. Cox proportional hazards regression models were employed. We next adjusted the models for age, sex, ethnicity, BMI, organ damage, baseline SLE disease activity, previous renal involvement, baseline prednisone equivalent dose, and use of immunosuppressants and belimumab.
Results: The mean age of the patients was 36.7 years, 94% were women, and 192 developed a renal flare after a median of 141 days. The proportion of patients with a history of renal involvement was 54.6%. In univariable Cox regression analysis, low C3 levels at baseline were associated with an increased risk to develop a renal flare (hazard ratio, HR: 2.6; 95% confidence interval, CI: 1.9–3.5; p< 0.001; figure), as did anti-dsDNA positivity (HR: 1.8; 95% CI: 1.3–2.6; p=0.001), anti-Sm positivity (HR: 1.6; 95% CI: 1.1–2.5; p=0.022), plasma albumin levels (HR: 0.9; 95% CI: 0.8–0.9; p< 0.001), and proteinuria (HR: 1.5; 95% CI: 1.4–1.6; p< 0.001). Low C4 levels were not associated with renal flare development. In adjusted analysis, the association with low C3 levels, plasma albumin and proteinuria remained significant.
Conclusion: Low C3, plasma albumin and proteinuria levels at baseline were associated with renal flare development in a clinical trial setting encompassing patients with active SLE yet no severe active LN, as were anti-dsDNA and anti-Sm positivity yet only in unadjusted models. While C3 and anti-dsDNA are markers of known importance for surveillance of renal SLE, anti-Sm and plasma albumin are not established markers of renal flare; investigation of potential complemental predictive properties across these traditional biomarkers has merit.

Disclosures: S. Jägerback, AstraZeneca; A. Gomez, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.

