Back
Abstract Session
Metabolic bone disease
Session: Abstracts: Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science (0574–0577)
0575: Myocardial Infarction and Stroke Risks Among Patients Who Initiated Treatment with Denosumab or Zoledronic Acid for Osteoporosis
Sunday, November 13, 2022
8:15 AM – 8:25 AM Eastern Time
Location: Room 113
- LS
Leslie Spangler, PhD, DVM
Amgen, Inc.
Thousand Oaks, CA, United States
Presenting Author(s)
Leslie Spangler1, Carrie Nielson1, M Alan Brookhart2, Rohini K Hernandez1, Robert Kees Stad3 and Jay Lin1, 1Amgen, Inc., Thousand Oaks, CA, 2NoviSci, Inc., Durham, NC, 3Amgen Inc., Zug, Switzerland
Background/Purpose: There has been inconsistent evidence published regarding the cardio- and cerebrovascular benefits and risks of anti-osteoporosis medications. EU regulators requested Amgen to evaluate the potential risk of these events in a real-world population of denosumab users treated for osteoporosis.
Methods: This was a retrospective, cohort study employing a comparative new user as-treated design conducted separately in two large US commercial claims databases (Optum and MarketScan®) among men and women (≥ 55 years of age) who initiated treatment with two different classes of antiresorptives, denosumab (RANKL inhibitor) or zoledronic acid (bisphosphonate) between 01 October 2010 and 30 June 2019. The risks of myocardial infarction (MI) and stroke (ischemic or hemorrhagic) were estimated at 6, 12, and 36 months using inverse probability of treatment and censoring weighting estimators to account for baseline differences in patient characteristics and for informative censoring.
Results: There were 73,127 patients in Optum (mean follow-up 350 days) and 96,611 in MarketScan (mean follow-up 385 days), with 90% female. A higher percent of patients treated with denosumab had chronic kidney disease (CKD), consistent with the caution around use of zoledronic acid in patients with chronic renal impairment. After adjustment, all measured baseline demographics and risk factors were comparable between treatment groups, including CKD stage (standardized mean differences < 0.10). There were 249 and 256 MI and 332 and 383 stroke events in Optum and MarketScan patients, respectively. At 36 months, the adjusted relative risks (zoledronic acid referent group) in Optum and MarketScan were 0.97 (95% CI: 0.63,1.32) and 1.22 (0.77,1.66) for MI, and 0.87 (0.56,1.17) and 1.00 (0.61,1.40) for stroke (Figure/Table). Similar results were observed when the stroke outcome was restricted to only ischemic stroke and when death was treated as a competing risk in the Optum analyses, where information on death was available. The slightly lower risk of MI at 6 and 12 months observed in denosumab users translated to a very small reduction in absolute risk and therefore was not considered to be clinically meaningful.
Conclusion: In a retrospective, cohort study conducted in two large US administrative claims data systems, no increased risk in MI or stroke was identified for up to 36 months of treatment in denosumab users compared with zoledronic acid users.
.jpg) Figure: Forest Plot of Adjusted Risk Ratios and 95% Confidence Intervals for Myocardial Infarction and Stroke at 6, 12, and 36 Months in Optum and MarketScan Cohorts
Figure: Forest Plot of Adjusted Risk Ratios and 95% Confidence Intervals for Myocardial Infarction and Stroke at 6, 12, and 36 Months in Optum and MarketScan Cohorts
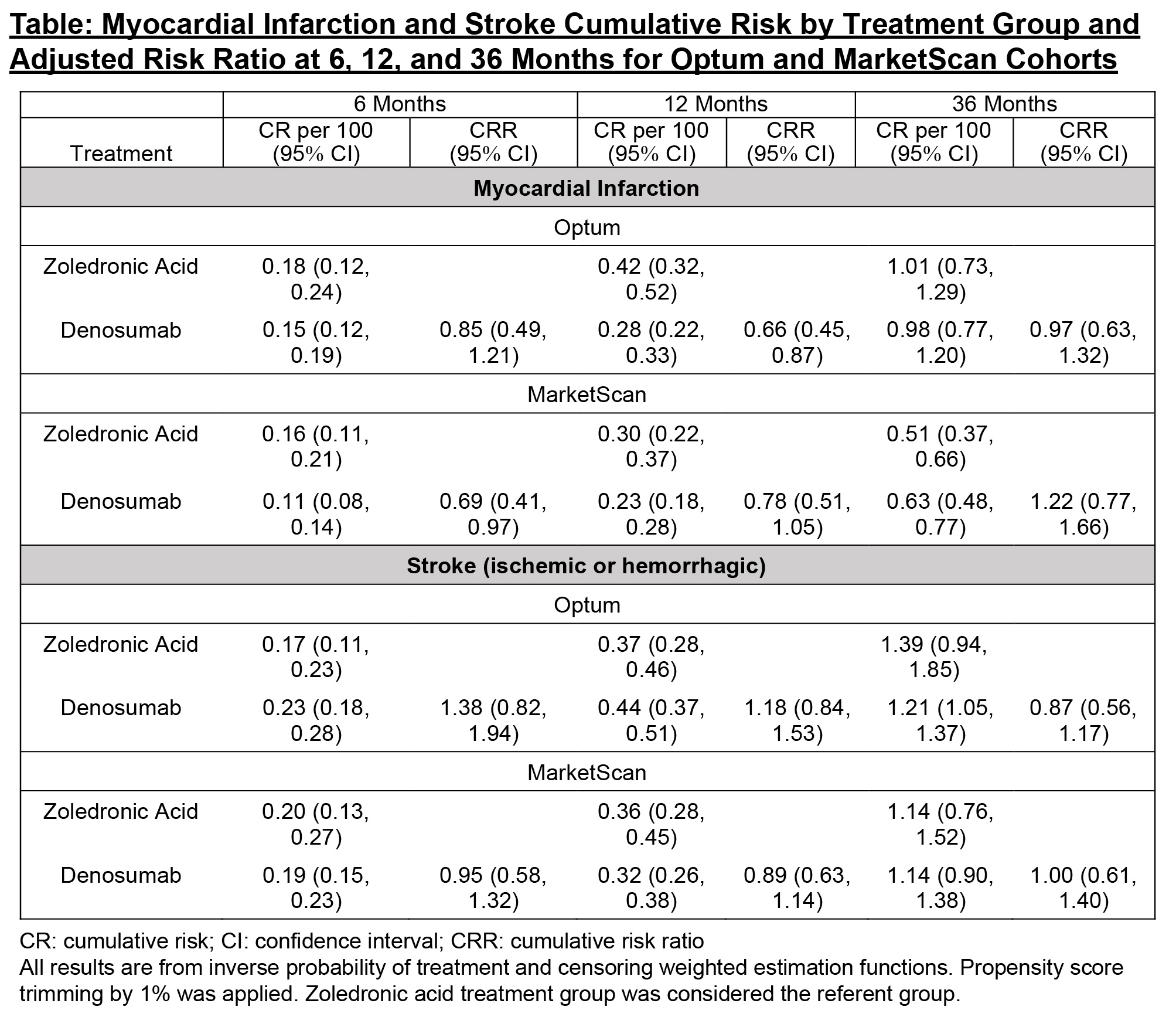 Table: Myocardial Infarction and Stroke Cumulative Risk by Treatment Group and Adjusted Risk Ratio at 6, 12, and 36 Months for Optum and MarketScan Cohorts
Table: Myocardial Infarction and Stroke Cumulative Risk by Treatment Group and Adjusted Risk Ratio at 6, 12, and 36 Months for Optum and MarketScan Cohorts
Disclosures: L. Spangler, Amgen; C. Nielson, Amgen; M. Brookhart, AbbVie/Abbott, Amgen, Gilead, Atara Biotherapeutics, Brigham and Women's Hospital, Kite; R. Hernandez, Amgen; R. Stad, Amgen; J. Lin, Amgen.
Background/Purpose: There has been inconsistent evidence published regarding the cardio- and cerebrovascular benefits and risks of anti-osteoporosis medications. EU regulators requested Amgen to evaluate the potential risk of these events in a real-world population of denosumab users treated for osteoporosis.
Methods: This was a retrospective, cohort study employing a comparative new user as-treated design conducted separately in two large US commercial claims databases (Optum and MarketScan®) among men and women (≥ 55 years of age) who initiated treatment with two different classes of antiresorptives, denosumab (RANKL inhibitor) or zoledronic acid (bisphosphonate) between 01 October 2010 and 30 June 2019. The risks of myocardial infarction (MI) and stroke (ischemic or hemorrhagic) were estimated at 6, 12, and 36 months using inverse probability of treatment and censoring weighting estimators to account for baseline differences in patient characteristics and for informative censoring.
Results: There were 73,127 patients in Optum (mean follow-up 350 days) and 96,611 in MarketScan (mean follow-up 385 days), with 90% female. A higher percent of patients treated with denosumab had chronic kidney disease (CKD), consistent with the caution around use of zoledronic acid in patients with chronic renal impairment. After adjustment, all measured baseline demographics and risk factors were comparable between treatment groups, including CKD stage (standardized mean differences < 0.10). There were 249 and 256 MI and 332 and 383 stroke events in Optum and MarketScan patients, respectively. At 36 months, the adjusted relative risks (zoledronic acid referent group) in Optum and MarketScan were 0.97 (95% CI: 0.63,1.32) and 1.22 (0.77,1.66) for MI, and 0.87 (0.56,1.17) and 1.00 (0.61,1.40) for stroke (Figure/Table). Similar results were observed when the stroke outcome was restricted to only ischemic stroke and when death was treated as a competing risk in the Optum analyses, where information on death was available. The slightly lower risk of MI at 6 and 12 months observed in denosumab users translated to a very small reduction in absolute risk and therefore was not considered to be clinically meaningful.
Conclusion: In a retrospective, cohort study conducted in two large US administrative claims data systems, no increased risk in MI or stroke was identified for up to 36 months of treatment in denosumab users compared with zoledronic acid users.
.jpg) Figure: Forest Plot of Adjusted Risk Ratios and 95% Confidence Intervals for Myocardial Infarction and Stroke at 6, 12, and 36 Months in Optum and MarketScan Cohorts
Figure: Forest Plot of Adjusted Risk Ratios and 95% Confidence Intervals for Myocardial Infarction and Stroke at 6, 12, and 36 Months in Optum and MarketScan Cohorts Table: Myocardial Infarction and Stroke Cumulative Risk by Treatment Group and Adjusted Risk Ratio at 6, 12, and 36 Months for Optum and MarketScan Cohorts
Table: Myocardial Infarction and Stroke Cumulative Risk by Treatment Group and Adjusted Risk Ratio at 6, 12, and 36 Months for Optum and MarketScan CohortsDisclosures: L. Spangler, Amgen; C. Nielson, Amgen; M. Brookhart, AbbVie/Abbott, Amgen, Gilead, Atara Biotherapeutics, Brigham and Women's Hospital, Kite; R. Hernandez, Amgen; R. Stad, Amgen; J. Lin, Amgen.

