Back
Abstract Session
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: Abstracts: Pediatric Rheumatology – Basic Science (0566–0569)
0569: Cytokine Storm Susceptibility Factors IL-18 and Cytotoxic Impairment Converge on Pathologic CD8 T-cell Hyperactivation
Sunday, November 13, 2022
8:45 AM – 8:55 AM Eastern Time
Location: Ballroom AB
.png)
Scott Canna, MD
Children's Hospital of Philadelphia
Philadelphia, PA, United States
Presenting Author(s)
Emily Landy1, Scott Canna2, Paul Tsoukas3, Vinh Dang2 and Jemy Varghese2, 1University of Pittsburgh, Pittsburgh, PA, 2Children's Hospital of Philadelphia, Philadelphia, PA, 3The Hospital for Sick Children, Toronto, ON, Canada
Background/Purpose: Mounting evidence suggests partial defects in cytotoxicity-related genes may promote hyperinflammation in Macrophage Activation Syndrome (MAS) patients, who uniformly have highly-elevated IL-18. In mice, neither excess IL-18 (18tg mice) nor perforin-deficiency individually cause immunopathology without inflammatory challenge. However,18tg mice lacking perforin (18tg;Prf1-/-) develop spontaneous lethal hyperinflammation, while subclinical MAS was found in 18tg;Prf1+/- mice. Recent studies in both HLH and MAS patients demonstrate CD8 T-cell activation profiles in peripheral blood. We sought to understand how these two susceptibility factors, IL-18 and cytotoxic impairment, synergize or converge on CD8 T cells to drive spontaneous hyperinflammation.
Methods: We bred and examined transgenic mice bearing single susceptibility and dual susceptibility factors to hyperinflammation (perforin deficiency and excess mature IL-18) by "clinical" measures, cytokine levels, flow cytometry, bulk RNA- and TCR-sequencing, and in functional studies.
Results: Flow cytometry analysis of dual susceptibility mice showed an expanded CD8 T-cells bearing high levels of the IL-18 receptor and multiple markers associated with exhaustion (PD-1, Lag-3, CD39), yet overproduce IFNg. Such "hyperinflammatory CD8 T-cells" are present in the reticuloendothelial organs (spleen, bone marrow, and liver) but not lymph nodes, peripheral blood, or thymus. Transcriptional analysis in splenic CD8 T-cells from dual susceptibility mice demonstrated that hyperactivated CD8 T cells reflect a pattern of activation distinct from acute effector, effector-memory, tissue-resident memory, or exhaustion when compared to well ascribed publicly available datasets. This analysis also revealed a strong signature for recent antigen activation. Bulk CD8 TCR sequencing showed dramatic oligoclonal hyper-expansion of TCR sequences from dual susceptibility mice as compared to controls with no significant clonal overlap between biologic replicate samples. Attempts to circumvent CD8 T-cell activation, using inducible deletion of Il18r1 only on CD8 T-cells, or by promoting allelic exclusion by fixing the T-cell receptor of DS mice, shows that hyperactivated CD8 T-cells can circumvent these efforts to hinder their ability to expand. In vitro, TCR stimulus is necessary for IL-18 to elicit its effects on yet does not inhibit activation- induced cell death.
Conclusion: IL-18 and cytotoxicity drive progressive and pathologic activation, post thymically, of a stochastic, oligoclonal CD8 T-cell population that underlies life-threatening immunopathology in HLH and MAS. Expansion of hyperactivated Cd8 T cells in reticuloendothelial organs curiously aligns with sites noted for high levels of hemophagocytosis. These data together suggest a requirement for TCR stimulation reminiscent of the infectious triggers common to HLH and MAS, demonstrate these cells remarkable resilience, and identify potentially-targetable nodes of T-cell activation.
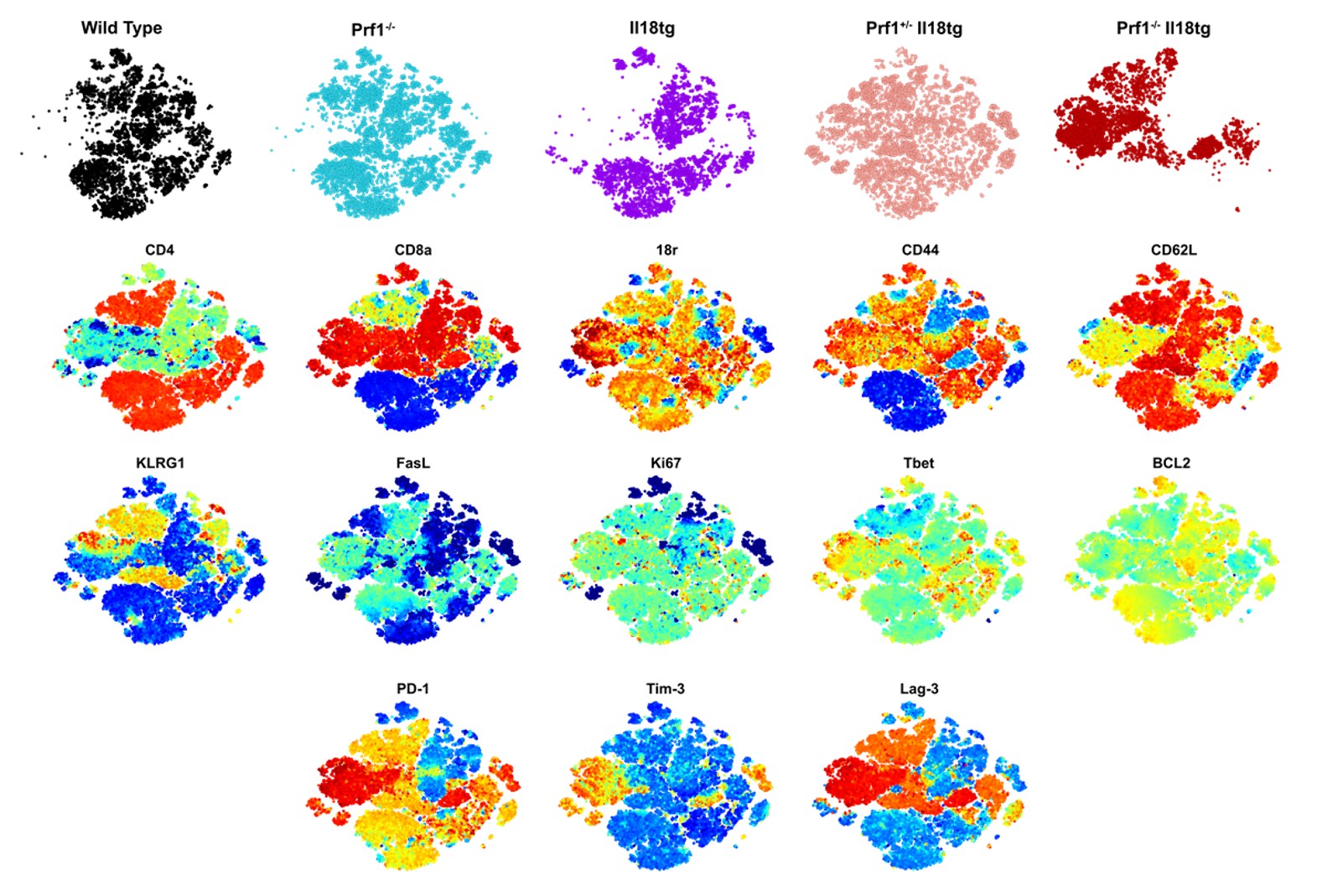 Flow analysis of T cells from mice with single and double susceptibility factors to hyperinflammation.
Flow analysis of T cells from mice with single and double susceptibility factors to hyperinflammation.
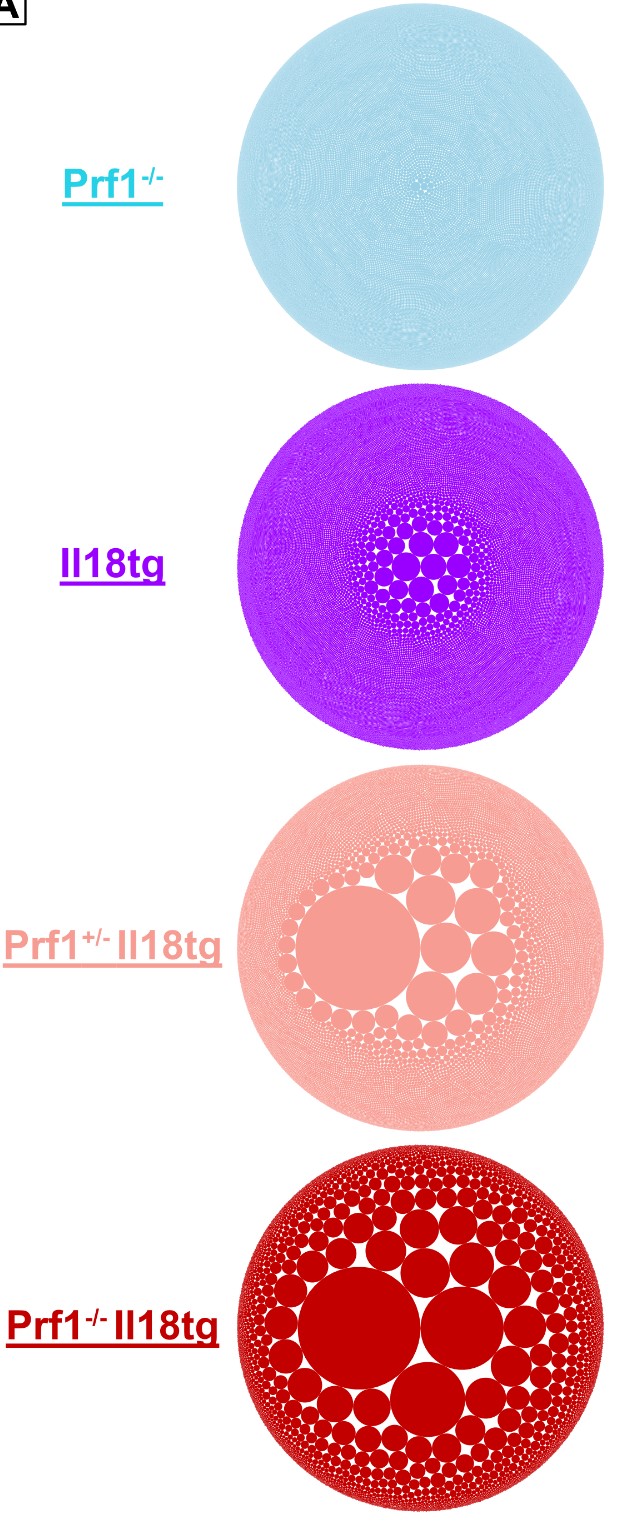 Bubble Plot representing the TCRb clonality within mice with single and double susceptibility factors to hyperinflammation. The bigger the bubble the more expanded that specific clone is.
Bubble Plot representing the TCRb clonality within mice with single and double susceptibility factors to hyperinflammation. The bigger the bubble the more expanded that specific clone is.
.jpg) Dual susceptibility hyperactivated CD8 T cells circumvent the pressure of allelic exclusion (fixing the T-cell receptor).
Dual susceptibility hyperactivated CD8 T cells circumvent the pressure of allelic exclusion (fixing the T-cell receptor).
Disclosures: E. Landy, None; S. Canna, Sobi, Ab2bio, Novartis, Immvention Therapeutics, sobi, Simcha Therapeutics; P. Tsoukas, None; V. Dang, None; J. Varghese, None.
Background/Purpose: Mounting evidence suggests partial defects in cytotoxicity-related genes may promote hyperinflammation in Macrophage Activation Syndrome (MAS) patients, who uniformly have highly-elevated IL-18. In mice, neither excess IL-18 (18tg mice) nor perforin-deficiency individually cause immunopathology without inflammatory challenge. However,18tg mice lacking perforin (18tg;Prf1-/-) develop spontaneous lethal hyperinflammation, while subclinical MAS was found in 18tg;Prf1+/- mice. Recent studies in both HLH and MAS patients demonstrate CD8 T-cell activation profiles in peripheral blood. We sought to understand how these two susceptibility factors, IL-18 and cytotoxic impairment, synergize or converge on CD8 T cells to drive spontaneous hyperinflammation.
Methods: We bred and examined transgenic mice bearing single susceptibility and dual susceptibility factors to hyperinflammation (perforin deficiency and excess mature IL-18) by "clinical" measures, cytokine levels, flow cytometry, bulk RNA- and TCR-sequencing, and in functional studies.
Results: Flow cytometry analysis of dual susceptibility mice showed an expanded CD8 T-cells bearing high levels of the IL-18 receptor and multiple markers associated with exhaustion (PD-1, Lag-3, CD39), yet overproduce IFNg. Such "hyperinflammatory CD8 T-cells" are present in the reticuloendothelial organs (spleen, bone marrow, and liver) but not lymph nodes, peripheral blood, or thymus. Transcriptional analysis in splenic CD8 T-cells from dual susceptibility mice demonstrated that hyperactivated CD8 T cells reflect a pattern of activation distinct from acute effector, effector-memory, tissue-resident memory, or exhaustion when compared to well ascribed publicly available datasets. This analysis also revealed a strong signature for recent antigen activation. Bulk CD8 TCR sequencing showed dramatic oligoclonal hyper-expansion of TCR sequences from dual susceptibility mice as compared to controls with no significant clonal overlap between biologic replicate samples. Attempts to circumvent CD8 T-cell activation, using inducible deletion of Il18r1 only on CD8 T-cells, or by promoting allelic exclusion by fixing the T-cell receptor of DS mice, shows that hyperactivated CD8 T-cells can circumvent these efforts to hinder their ability to expand. In vitro, TCR stimulus is necessary for IL-18 to elicit its effects on yet does not inhibit activation- induced cell death.
Conclusion: IL-18 and cytotoxicity drive progressive and pathologic activation, post thymically, of a stochastic, oligoclonal CD8 T-cell population that underlies life-threatening immunopathology in HLH and MAS. Expansion of hyperactivated Cd8 T cells in reticuloendothelial organs curiously aligns with sites noted for high levels of hemophagocytosis. These data together suggest a requirement for TCR stimulation reminiscent of the infectious triggers common to HLH and MAS, demonstrate these cells remarkable resilience, and identify potentially-targetable nodes of T-cell activation.
 Flow analysis of T cells from mice with single and double susceptibility factors to hyperinflammation.
Flow analysis of T cells from mice with single and double susceptibility factors to hyperinflammation. Bubble Plot representing the TCRb clonality within mice with single and double susceptibility factors to hyperinflammation. The bigger the bubble the more expanded that specific clone is.
Bubble Plot representing the TCRb clonality within mice with single and double susceptibility factors to hyperinflammation. The bigger the bubble the more expanded that specific clone is. .jpg) Dual susceptibility hyperactivated CD8 T cells circumvent the pressure of allelic exclusion (fixing the T-cell receptor).
Dual susceptibility hyperactivated CD8 T cells circumvent the pressure of allelic exclusion (fixing the T-cell receptor). Disclosures: E. Landy, None; S. Canna, Sobi, Ab2bio, Novartis, Immvention Therapeutics, sobi, Simcha Therapeutics; P. Tsoukas, None; V. Dang, None; J. Varghese, None.

