Back
Poster Session D
Immunobiology
Session: (1681–1706) Innate Immunity Poster: Basic and Translational Science
1705: Peptidoglycan Amplifies CD4+ T Cell Activation by MHCII+ Fibroblast-like Synoviocytes in an in Vitro Model of Lyme Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RL
Robert Lochhead, PhD
Medical College of Wisconsin
GERMANTOWN, WI, United States
Abstract Poster Presenter(s)
Joseph Rouse1, Amanda Wahhab1, Rebecca Danner1, Brandon Jutras2, Adam Edelstein3, Klemen Strle4 and Robert Lochhead5, 1Medical College of Wisconsin, Milwaukee, WI, 2Virginia Tech, Blacksburg, VA, 3Northwestern Medicine, Chicago, IL, 4Wadsworth Center, Albany, NY, 5Medical College of Wisconsin, Germantown, WI
Background/Purpose: Lyme arthritis (LA), caused by infection with Borrelia burgdorferi (Bb), is characterized by proliferative synovitis accompanied by dysregulated autoimmune Th1 responses. Fibroblast-like synoviocytes (FLS) are the most abundant cell in the synovial lesion and express markers of immune activation, including MHC class II molecules. Bb cell wall peptidoglycan (PG) is a persistent pathogen-associated molecular pattern (PAMP) in joints of LA patients and persists months to several years after antibiotic therapy and apparent spirochetal killing, called post-infectious LA. We hypothesize that PG acts as an immune adjuvant to activate MHCII+ FLS and enhance Th1 activation in synovial tissue during infection and in post-infectious LA.
Methods: FLS were isolated and purified from tibiotarsal ankles of C57BL/6 mice and stimulated with IFN-gamma, PG, or both. Wild-type macrophages and macrophages and FLS from mice deficient in the PG-sensing pattern recognition receptor NOD2 were used as controls. Immune responses were measured by flow cytometry and cytokine analyses. T cell activation was analyzed by coculturing MHCII+ FLS with CFSE-labeled naive OT-II T cells supplemented with the cognate OVA323-339 peptide or irrelevant OVA257-264 peptide as control. T cell activation was measured using a proliferation assay and T cell cytokine analysis.
Results: FLS stimulated with IFN-gamma expressed MHCII and pro-inflammatory cytokines IL-1, IL-6, and IL-12, consistent with our previous results using primary human FLS. FLS stimulated with both PG and IFN-gamma expressed significantly higher levels of MHCII and inflammatory cytokines, compared with IFN-gamma stimulation alone (Figure 1). Naive OT-II T cells, which only recognize the MHCII peptide OVA323-339, proliferated 3-fold compared with media alone control when co-cultured with FLS primed with IFN-gamma and supplemented with the OVA323-339 peptide. FLS supplemented with the irrelevant OVA257-264 peptide did not elicit OT-II T cell proliferation. FLS primed with both IFN-gamma and PG induced a 5-fold increase in OT-II proliferation, compared with media alone control. T cell supernatants contained high levels of IFN-gamma, indicating Th1 polarization of activated CD4+ T cells. FLS lacking NOD2 failed to induce CD4+ T cell proliferation (Figure 2).
Conclusion: These data demonstrate that PG acts as an immune adjuvant by enhancing the ability of MHCII+ FLS to induce CD4+ T cell proliferation in a peptide-dependent and NOD2-dependent manner. This response recapitulates the autoimmune Th1 responses observed in synovial tissue of post-infectious LA patients. This study helps us understand the critical role MHCII+ FLS and bacterial cell wall debris play in driving persistent inflammatory T cell responses within the synovial lesion, even in the absence of active infection. These results also shed light on development of autoimmunity in other inflammatory joint diseases where bacteria are thought to be an autoimmune trigger.
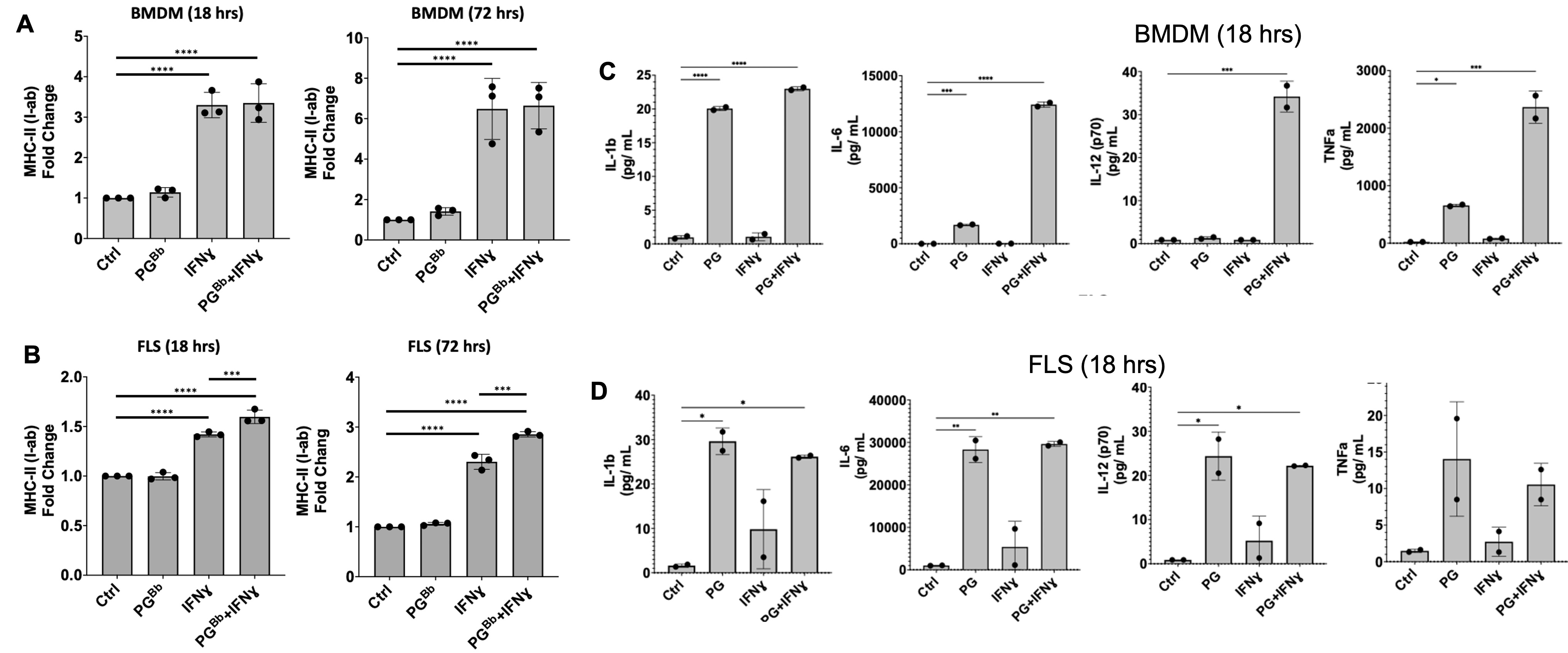 Figure 1: FLS immune effector phenotype in response to PG. B6 mouse BMDM and FLS surface expression of MHC-II (I-ab in mice) (left) and proinflammatory cytokine production (right) when stimulated with IFNɣ, PG, or both. (A) BMDM upregulated MHC-II surface expression in an IFNɣ-dependent manner, as expected. (B) FLS primed with IFNɣ upregulate MHC-II when stimulated with IFNɣ and B. burgdorferi PG for 18 hrs and this response was strengthened after prolonged exposure at 72 hrs. (C) BMDM and (D) FLS upregulation of proinflammatory cytokines in response to stimulation with PG muropeptides for 18 hrs. ***P < 0.0001, **P < 0.001, *P < 0.01
Figure 1: FLS immune effector phenotype in response to PG. B6 mouse BMDM and FLS surface expression of MHC-II (I-ab in mice) (left) and proinflammatory cytokine production (right) when stimulated with IFNɣ, PG, or both. (A) BMDM upregulated MHC-II surface expression in an IFNɣ-dependent manner, as expected. (B) FLS primed with IFNɣ upregulate MHC-II when stimulated with IFNɣ and B. burgdorferi PG for 18 hrs and this response was strengthened after prolonged exposure at 72 hrs. (C) BMDM and (D) FLS upregulation of proinflammatory cytokines in response to stimulation with PG muropeptides for 18 hrs. ***P < 0.0001, **P < 0.001, *P < 0.01
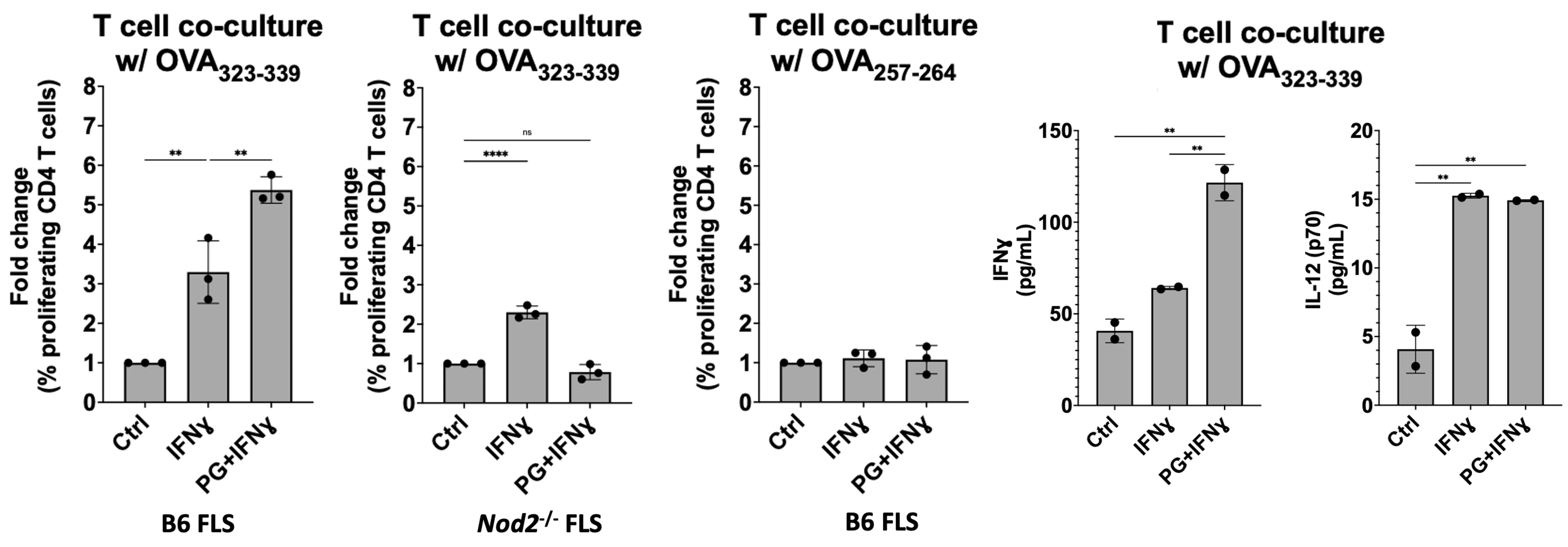 Figure 2: MHC-II+ FLS activate peptide-specific CD4+ T cell proliferation. IFNɣ-primed MHC-II+ B6 FLS were co-cultured with OT-II mouse T cells (genetically modified to have identical antigen-specific T cell receptors) and loaded with the cognate OVA323-339 peptide. T cell responses measured by flow cytometry and multiplex immunoassay. T cell co-culture with MHC-II+ FLS primed with IFNɣ induced naive OT-II mouse CD4+ T cell proliferation at a 3-fold increase compared to unstimulated FLS, which was enhanced to above 5-fold when co-cultured with FLS primed with both IFNɣ and PG. Negative controls using irrelevant OVA peptide or NOD2-deficient FLS failed to induce T cell proliferation.
Figure 2: MHC-II+ FLS activate peptide-specific CD4+ T cell proliferation. IFNɣ-primed MHC-II+ B6 FLS were co-cultured with OT-II mouse T cells (genetically modified to have identical antigen-specific T cell receptors) and loaded with the cognate OVA323-339 peptide. T cell responses measured by flow cytometry and multiplex immunoassay. T cell co-culture with MHC-II+ FLS primed with IFNɣ induced naive OT-II mouse CD4+ T cell proliferation at a 3-fold increase compared to unstimulated FLS, which was enhanced to above 5-fold when co-cultured with FLS primed with both IFNɣ and PG. Negative controls using irrelevant OVA peptide or NOD2-deficient FLS failed to induce T cell proliferation.
Disclosures: J. Rouse, None; A. Wahhab, None; R. Danner, None; B. Jutras, None; A. Edelstein, None; K. Strle, None; R. Lochhead, None.
Background/Purpose: Lyme arthritis (LA), caused by infection with Borrelia burgdorferi (Bb), is characterized by proliferative synovitis accompanied by dysregulated autoimmune Th1 responses. Fibroblast-like synoviocytes (FLS) are the most abundant cell in the synovial lesion and express markers of immune activation, including MHC class II molecules. Bb cell wall peptidoglycan (PG) is a persistent pathogen-associated molecular pattern (PAMP) in joints of LA patients and persists months to several years after antibiotic therapy and apparent spirochetal killing, called post-infectious LA. We hypothesize that PG acts as an immune adjuvant to activate MHCII+ FLS and enhance Th1 activation in synovial tissue during infection and in post-infectious LA.
Methods: FLS were isolated and purified from tibiotarsal ankles of C57BL/6 mice and stimulated with IFN-gamma, PG, or both. Wild-type macrophages and macrophages and FLS from mice deficient in the PG-sensing pattern recognition receptor NOD2 were used as controls. Immune responses were measured by flow cytometry and cytokine analyses. T cell activation was analyzed by coculturing MHCII+ FLS with CFSE-labeled naive OT-II T cells supplemented with the cognate OVA323-339 peptide or irrelevant OVA257-264 peptide as control. T cell activation was measured using a proliferation assay and T cell cytokine analysis.
Results: FLS stimulated with IFN-gamma expressed MHCII and pro-inflammatory cytokines IL-1, IL-6, and IL-12, consistent with our previous results using primary human FLS. FLS stimulated with both PG and IFN-gamma expressed significantly higher levels of MHCII and inflammatory cytokines, compared with IFN-gamma stimulation alone (Figure 1). Naive OT-II T cells, which only recognize the MHCII peptide OVA323-339, proliferated 3-fold compared with media alone control when co-cultured with FLS primed with IFN-gamma and supplemented with the OVA323-339 peptide. FLS supplemented with the irrelevant OVA257-264 peptide did not elicit OT-II T cell proliferation. FLS primed with both IFN-gamma and PG induced a 5-fold increase in OT-II proliferation, compared with media alone control. T cell supernatants contained high levels of IFN-gamma, indicating Th1 polarization of activated CD4+ T cells. FLS lacking NOD2 failed to induce CD4+ T cell proliferation (Figure 2).
Conclusion: These data demonstrate that PG acts as an immune adjuvant by enhancing the ability of MHCII+ FLS to induce CD4+ T cell proliferation in a peptide-dependent and NOD2-dependent manner. This response recapitulates the autoimmune Th1 responses observed in synovial tissue of post-infectious LA patients. This study helps us understand the critical role MHCII+ FLS and bacterial cell wall debris play in driving persistent inflammatory T cell responses within the synovial lesion, even in the absence of active infection. These results also shed light on development of autoimmunity in other inflammatory joint diseases where bacteria are thought to be an autoimmune trigger.
 Figure 1: FLS immune effector phenotype in response to PG. B6 mouse BMDM and FLS surface expression of MHC-II (I-ab in mice) (left) and proinflammatory cytokine production (right) when stimulated with IFNɣ, PG, or both. (A) BMDM upregulated MHC-II surface expression in an IFNɣ-dependent manner, as expected. (B) FLS primed with IFNɣ upregulate MHC-II when stimulated with IFNɣ and B. burgdorferi PG for 18 hrs and this response was strengthened after prolonged exposure at 72 hrs. (C) BMDM and (D) FLS upregulation of proinflammatory cytokines in response to stimulation with PG muropeptides for 18 hrs. ***P < 0.0001, **P < 0.001, *P < 0.01
Figure 1: FLS immune effector phenotype in response to PG. B6 mouse BMDM and FLS surface expression of MHC-II (I-ab in mice) (left) and proinflammatory cytokine production (right) when stimulated with IFNɣ, PG, or both. (A) BMDM upregulated MHC-II surface expression in an IFNɣ-dependent manner, as expected. (B) FLS primed with IFNɣ upregulate MHC-II when stimulated with IFNɣ and B. burgdorferi PG for 18 hrs and this response was strengthened after prolonged exposure at 72 hrs. (C) BMDM and (D) FLS upregulation of proinflammatory cytokines in response to stimulation with PG muropeptides for 18 hrs. ***P < 0.0001, **P < 0.001, *P < 0.01 Figure 2: MHC-II+ FLS activate peptide-specific CD4+ T cell proliferation. IFNɣ-primed MHC-II+ B6 FLS were co-cultured with OT-II mouse T cells (genetically modified to have identical antigen-specific T cell receptors) and loaded with the cognate OVA323-339 peptide. T cell responses measured by flow cytometry and multiplex immunoassay. T cell co-culture with MHC-II+ FLS primed with IFNɣ induced naive OT-II mouse CD4+ T cell proliferation at a 3-fold increase compared to unstimulated FLS, which was enhanced to above 5-fold when co-cultured with FLS primed with both IFNɣ and PG. Negative controls using irrelevant OVA peptide or NOD2-deficient FLS failed to induce T cell proliferation.
Figure 2: MHC-II+ FLS activate peptide-specific CD4+ T cell proliferation. IFNɣ-primed MHC-II+ B6 FLS were co-cultured with OT-II mouse T cells (genetically modified to have identical antigen-specific T cell receptors) and loaded with the cognate OVA323-339 peptide. T cell responses measured by flow cytometry and multiplex immunoassay. T cell co-culture with MHC-II+ FLS primed with IFNɣ induced naive OT-II mouse CD4+ T cell proliferation at a 3-fold increase compared to unstimulated FLS, which was enhanced to above 5-fold when co-cultured with FLS primed with both IFNɣ and PG. Negative controls using irrelevant OVA peptide or NOD2-deficient FLS failed to induce T cell proliferation.Disclosures: J. Rouse, None; A. Wahhab, None; R. Danner, None; B. Jutras, None; A. Edelstein, None; K. Strle, None; R. Lochhead, None.

