Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0598: An Arthritogenic Strain of Subdoligranulum Specifically Detectable in the Feces of Individuals At-risk for and with Rheumatoid Arthritis Is Bound by ACPA and Stimulates Th17 Cell Activation in Those with Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MC
Meagan Chriswell, MD, PhD
UC Denver SOM
Denver, CO, United States
Abstract Poster Presenter(s)
Meagan Chriswell1, Cliff Rims2, Megan Maerz2, Alex Hsu3, Jennifer Seifert4, Marie Feser5, Michelle Bloom3, Elizabeth Bemis6, Kristen Demoruelle5, Kevin D Deane7, William Robinson8, Eddie James9, Jane Buckner9, V. Michael Holers10 and Kristi Kuhn5, 1UC Denver SOM, Aurora, CO, 2Benaroya Research Institute, Seattle, WA, 3Stanford University, Stanford, CA, 4University of Colorado, Littleton, CO, 5University of Colorado Anschutz Medical Campus, Aurora, CO, 6Colorado School of Public Health Anschutz Medical Campus, Aurora, CO, 7University of Colorado Denver Anschutz Medical Campus, Denver, CO, 8Stanford University School of Medicine, Palo Alto, CA, 9Benaroya Research Institute at Virginia Mason, Seattle, WA, 10University of Colorado, Denver, CO
Background/Purpose: We previously demonstrated that intestinal bacteria within the families Lachnospiraceae and Ruminococcaceae are preferentially targeted by ACPA-reactive plasmablast-derived monoclonal antibodies (PB-mAbs) from dual IgA/IgG isotype families derived from individuals at-risk for RA. Furthermore, intestinal colonization of mice with a PB-mAb reactive primary Ruminococcaceae genus Subdoligranulum strain, designated strain 7, from an at-risk individual drove Th17 cell responses as well as serum RA-related autoantibodies and inflammatory joint swelling in monocolonized mice in the absence of other arthritogenic factors. Development of arthritis required B and T cells in mice, and could be transferred with serum to naïve mice. Given these findings, we hypothesized that the presence of this arthritogenic Subdoligranulum strain 7 is specific to those at risk for and with RA and that a specific anti-strain 7 immune response is found in human RA.
Methods: Feces from individuals at-risk for (n=12, defined as being serum anti-CCP3 positive) and with early RA (n=12, < 1 year from diagnosis), as well as healthy controls (n=12), were screened for the presence of Subdoligranulum strain 7 by a strain-specific qPCR with a sensitivity of 0.0002% abundance in feces. Human PBMCs from individuals with early RA (n=11) and healthy controls (n=12) were exposed to strains 1 and 7 as well as influenza, and T cell activation based on CD69 and CD154 upregulation were evaluated by flow cytometry. CD45RA-/CXCR3+/CCR4-/CCR6- cell markers were used as to define Th1 cells while CD45RA-/CXCR3-/CCR4+/CCR6+ were used for Th17 cells.
Results: Subdoligranulum strain 7 was detectable in the feces at a mean abundance 1.637 x 107± 3.059 x 107 CFUs in ~16% of individuals at-risk for and with early RA, but not detected in healthy individuals (Table 1). ACPA-reactive PB-mAbs bound strains 1 and 7, and digestion of cell wall associated proteins with 100 µg/mL Proteinase K decreased binding by 48%. In addition, informative PB-mAbs could immunoprecipitate a molecule of 10 kD from a cell wall-enriched fraction in strain 7 but not strain 1. Finally, strain 7 but not strain 1 induced T cell activation in human PBMCs from individuals with RA, but not controls (Fig. 1, P< 0.01). This response was HLA class II dependent and skewed towards a Th17 phenotype, as compared to influenza that induced a Th1-like response (P=0.0078, Fig. 2).
Conclusion: The preferential presence of, and immune responses against, a primary human-derived strain of Subdoligranulum in individuals at-risk for or with early RA, but not in controls, supports the conclusion that this is an arthritogenic bacterium of relevance in a subset of RA risk and patient populations. The finding of PB-mAb-reactive antigen associated with the cell wall fraction of Subdoligranulum, suggests that a cross-reactive protein may be stimulating systemic adaptive immunity in RA through molecular mimicry. Taken alongside our murine findings, these data implicate Subdoligranulum strain 7 as a candidate mucosal driver of RA development in a subset of individuals.
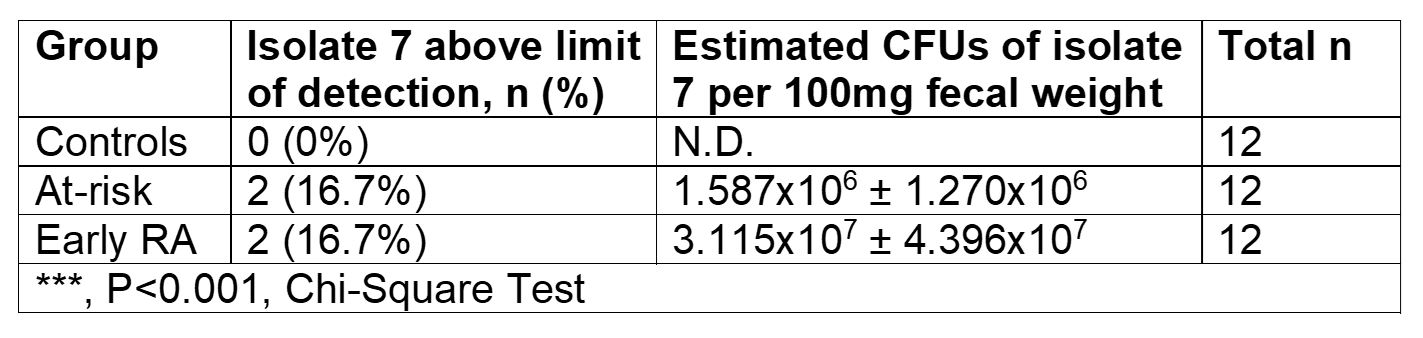
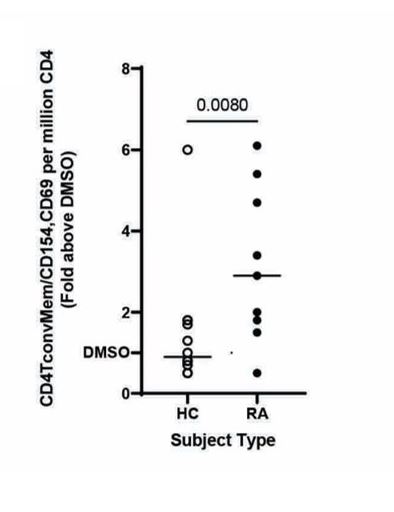 Fig. 1: PBMCs from RA cases (n=11) or controls (n=12) were stimulated with 50ng/ml strain 7. Fold change of the CD4 T cell activation based on CD154 and CD69 surface expression relative to DMSO is displayed. (p=0.0080, nonparametric Mann-Whitney test)
Fig. 1: PBMCs from RA cases (n=11) or controls (n=12) were stimulated with 50ng/ml strain 7. Fold change of the CD4 T cell activation based on CD154 and CD69 surface expression relative to DMSO is displayed. (p=0.0080, nonparametric Mann-Whitney test)
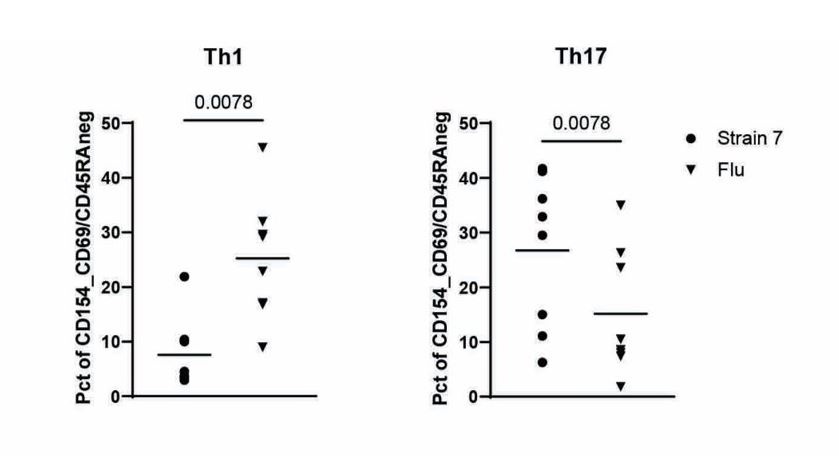 Fig 2.: Using CD45RA-/CXCR3+/CCR4-/CCR6- (Th1) or CD45RA-/CXCR3-/CCR4+/CCR6+ (Th17) as a defining marker combinations, we compared the relative proportion of Th1- and Th17-like cells for strain 7 specific (circles) or influenza specific (inverted triangles) memory T cell responses in T cells from RA cases (p=0.0078, nonparametric Mann-Whitney test).
Fig 2.: Using CD45RA-/CXCR3+/CCR4-/CCR6- (Th1) or CD45RA-/CXCR3-/CCR4+/CCR6+ (Th17) as a defining marker combinations, we compared the relative proportion of Th1- and Th17-like cells for strain 7 specific (circles) or influenza specific (inverted triangles) memory T cell responses in T cells from RA cases (p=0.0078, nonparametric Mann-Whitney test).
Disclosures: M. Chriswell, None; C. Rims, None; M. Maerz, None; A. Hsu, None; J. Seifert, None; M. Feser, None; M. Bloom, None; E. Bemis, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; K. Deane, Werfen; W. Robinson, None; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; V. Holers, Janssen; K. Kuhn, None.
Background/Purpose: We previously demonstrated that intestinal bacteria within the families Lachnospiraceae and Ruminococcaceae are preferentially targeted by ACPA-reactive plasmablast-derived monoclonal antibodies (PB-mAbs) from dual IgA/IgG isotype families derived from individuals at-risk for RA. Furthermore, intestinal colonization of mice with a PB-mAb reactive primary Ruminococcaceae genus Subdoligranulum strain, designated strain 7, from an at-risk individual drove Th17 cell responses as well as serum RA-related autoantibodies and inflammatory joint swelling in monocolonized mice in the absence of other arthritogenic factors. Development of arthritis required B and T cells in mice, and could be transferred with serum to naïve mice. Given these findings, we hypothesized that the presence of this arthritogenic Subdoligranulum strain 7 is specific to those at risk for and with RA and that a specific anti-strain 7 immune response is found in human RA.
Methods: Feces from individuals at-risk for (n=12, defined as being serum anti-CCP3 positive) and with early RA (n=12, < 1 year from diagnosis), as well as healthy controls (n=12), were screened for the presence of Subdoligranulum strain 7 by a strain-specific qPCR with a sensitivity of 0.0002% abundance in feces. Human PBMCs from individuals with early RA (n=11) and healthy controls (n=12) were exposed to strains 1 and 7 as well as influenza, and T cell activation based on CD69 and CD154 upregulation were evaluated by flow cytometry. CD45RA-/CXCR3+/CCR4-/CCR6- cell markers were used as to define Th1 cells while CD45RA-/CXCR3-/CCR4+/CCR6+ were used for Th17 cells.
Results: Subdoligranulum strain 7 was detectable in the feces at a mean abundance 1.637 x 107± 3.059 x 107 CFUs in ~16% of individuals at-risk for and with early RA, but not detected in healthy individuals (Table 1). ACPA-reactive PB-mAbs bound strains 1 and 7, and digestion of cell wall associated proteins with 100 µg/mL Proteinase K decreased binding by 48%. In addition, informative PB-mAbs could immunoprecipitate a molecule of 10 kD from a cell wall-enriched fraction in strain 7 but not strain 1. Finally, strain 7 but not strain 1 induced T cell activation in human PBMCs from individuals with RA, but not controls (Fig. 1, P< 0.01). This response was HLA class II dependent and skewed towards a Th17 phenotype, as compared to influenza that induced a Th1-like response (P=0.0078, Fig. 2).
Conclusion: The preferential presence of, and immune responses against, a primary human-derived strain of Subdoligranulum in individuals at-risk for or with early RA, but not in controls, supports the conclusion that this is an arthritogenic bacterium of relevance in a subset of RA risk and patient populations. The finding of PB-mAb-reactive antigen associated with the cell wall fraction of Subdoligranulum, suggests that a cross-reactive protein may be stimulating systemic adaptive immunity in RA through molecular mimicry. Taken alongside our murine findings, these data implicate Subdoligranulum strain 7 as a candidate mucosal driver of RA development in a subset of individuals.

 Fig. 1: PBMCs from RA cases (n=11) or controls (n=12) were stimulated with 50ng/ml strain 7. Fold change of the CD4 T cell activation based on CD154 and CD69 surface expression relative to DMSO is displayed. (p=0.0080, nonparametric Mann-Whitney test)
Fig. 1: PBMCs from RA cases (n=11) or controls (n=12) were stimulated with 50ng/ml strain 7. Fold change of the CD4 T cell activation based on CD154 and CD69 surface expression relative to DMSO is displayed. (p=0.0080, nonparametric Mann-Whitney test)  Fig 2.: Using CD45RA-/CXCR3+/CCR4-/CCR6- (Th1) or CD45RA-/CXCR3-/CCR4+/CCR6+ (Th17) as a defining marker combinations, we compared the relative proportion of Th1- and Th17-like cells for strain 7 specific (circles) or influenza specific (inverted triangles) memory T cell responses in T cells from RA cases (p=0.0078, nonparametric Mann-Whitney test).
Fig 2.: Using CD45RA-/CXCR3+/CCR4-/CCR6- (Th1) or CD45RA-/CXCR3-/CCR4+/CCR6+ (Th17) as a defining marker combinations, we compared the relative proportion of Th1- and Th17-like cells for strain 7 specific (circles) or influenza specific (inverted triangles) memory T cell responses in T cells from RA cases (p=0.0078, nonparametric Mann-Whitney test). Disclosures: M. Chriswell, None; C. Rims, None; M. Maerz, None; A. Hsu, None; J. Seifert, None; M. Feser, None; M. Bloom, None; E. Bemis, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; K. Deane, Werfen; W. Robinson, None; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; V. Holers, Janssen; K. Kuhn, None.

