Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1804: Raman Spectroscopy Integrated with Polarized Light Microscopy for Diagnosis of Crystallopathies
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- TN
Tom Niessink, MSc
University of Twente
Utrecht, Netherlands
Abstract Poster Presenter(s)
Tom Niessink1, Matthijs Janssen2, Cees Otto1 and Tim Jansen3, 1Medical Cell Biophysics group, University of Twente, Enschede, Netherlands, 2Rijnstate Hospital, Bennekom, Netherlands, 3Department of Rheumatology, VieCuri Medical Centre, Venlo, Netherlands
Background/Purpose: The current golden standard in diagnosing gout and calcium pyrophosphate deposition is polarized light microscopy (PLM). However, small crystal sizes, the presence of other crystal types, glass artefacts and cellular uptake of MSU can pose uncertainties in the clinical analysis of synovial fluids. There are multiple methods to improve synovial fluid analysis, of which Raman spectroscopy is the most promising. In our approach, we have integrated an ordinary polarized light microscope with a Raman spectroscope (iRPolM, Hybriscan), allowing for simultaneous use of both techniques. In this abstract, we demonstrate the added value of this technique for the first 50 patients, including 23 diagnosed with gout.
Methods: 50 patients were punctured for diagnostic purposes when presenting with swollen joints. Patient characteristics are given in figure 1. Samples were analyzed by an experienced rheumatologist with polarization microscopy, who also gave an indication of certainty in diagnosis. The iRPolM analysis followed this. A visual representation of this method can be found in figure 2. Retrieved Raman spectra were analyzed by a trained Raman spectroscopist for the presence of MSU, calcium pyrophosphate dihydrate (CPPD) or other (deposited) materials.
Results: A summary of the results from this experiment can be found in figure 3. We demonstrate a high correspondence (95%) between a certain identification with PLM and iRPolM. While the rheumatologist is only able to identify MSU/CPPD, Raman can also discriminate other microparticles.
Hydroxyapatite crystals were found in 1 patient with RA and one patient with a degenerative shoulder syndrome. Calcite (calcium carbonate) could be identified in 17 patients, 4 of whom were diagnosed with gout, 4 with CPPD, 3 with Rheumatoid Arthritis, 2 with Osteoarthritis and 4 undefined.
Titanium dioxide crystals were found in 6 patients. Only one of 6 patients with titanium crystals had an orthopedic implant, there is no clear relation between the two.
Carotenoids, a precursor molecule for vitamin A, could be found in 4 patients, 1 of which was diagnosed with gout and 3 had an undefined joint disease. All thenardite (sodium sulphate) crystals could be found in patients with osteoarthritis. Microplastics could be found in 19 patients, but environmental pollution must be excluded.
Conclusion: Raman spectroscopy has a significant added value to PLM, as it can not only can discriminate between MSU and CPPD but also identify a whole range of other crystals which might be related to potentially novel crystallopathies. In the future crystal identification in rheumatology may well have to rely on Raman based diagnostics.
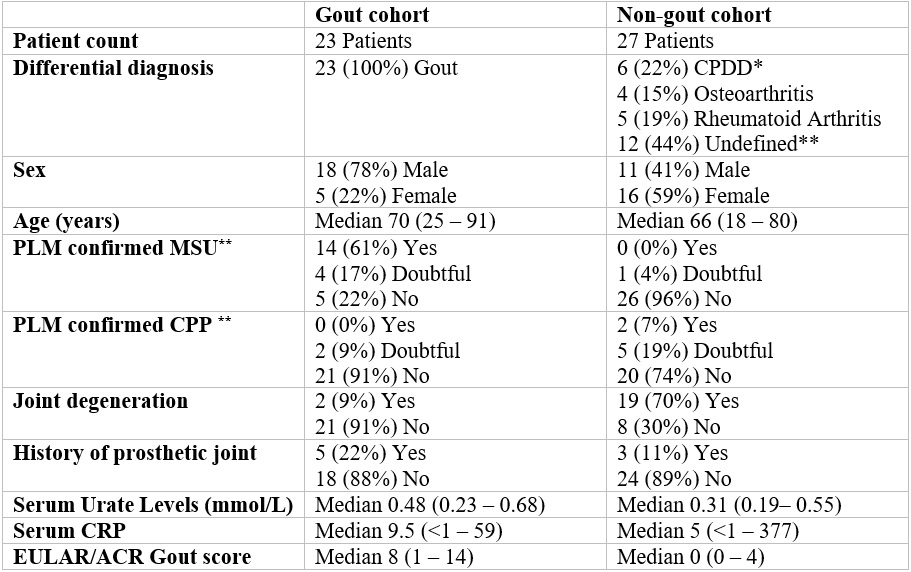 Patient characteristics table. * CPDD: calcium pyrophosphate deposition disease ** Undefined means joint disease other than gout, CPPD, rheumatoid arthritis or osteoarthritis. *** Yes/no means a 100% certain crystal identification as assessed by an experienced rheumatologist. Doubtful means a less than 100% certain crystal identification
Patient characteristics table. * CPDD: calcium pyrophosphate deposition disease ** Undefined means joint disease other than gout, CPPD, rheumatoid arthritis or osteoarthritis. *** Yes/no means a 100% certain crystal identification as assessed by an experienced rheumatologist. Doubtful means a less than 100% certain crystal identification
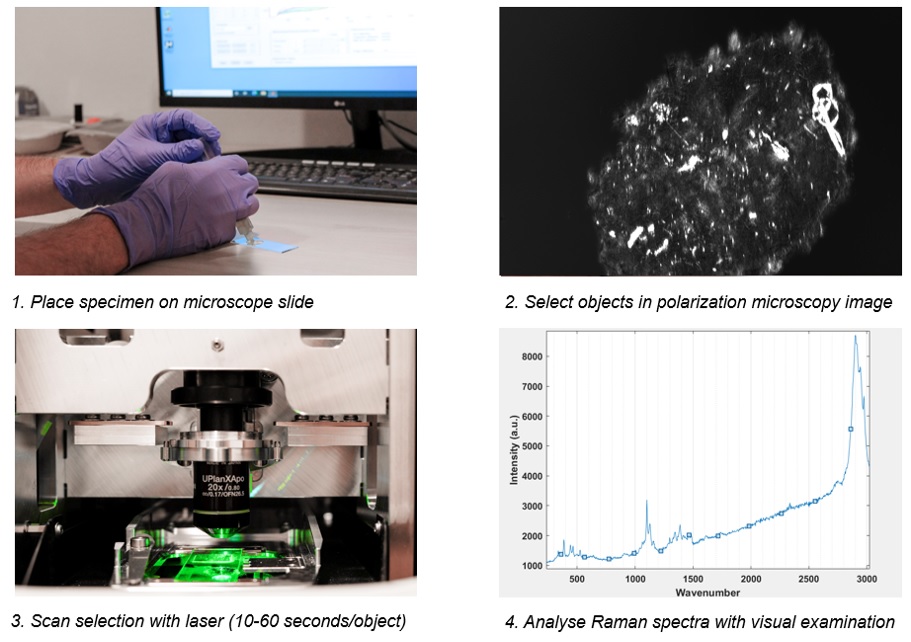 Graphic depiction of the Raman analysis process. After scanning, Raman spectra are analyzed with visual examination by a trained spectroscopist. The presence of a peak at 630 wavenumber (cm-1) is specific for MSU crystals, whereas a peak at 1050 wavenumber (cm-1) is specific for calcium pyrophosphate crystals.
Graphic depiction of the Raman analysis process. After scanning, Raman spectra are analyzed with visual examination by a trained spectroscopist. The presence of a peak at 630 wavenumber (cm-1) is specific for MSU crystals, whereas a peak at 1050 wavenumber (cm-1) is specific for calcium pyrophosphate crystals.
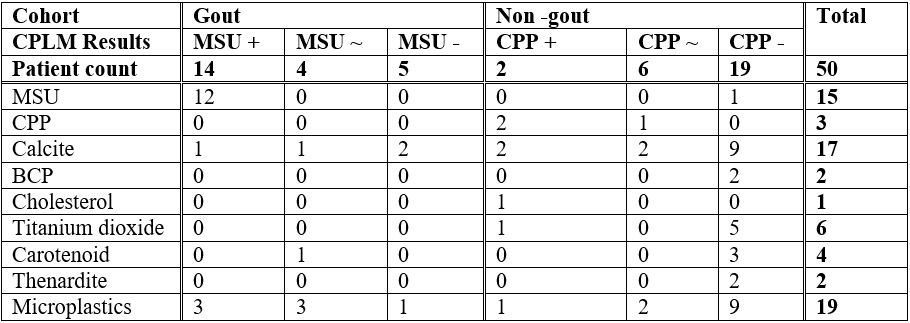 Results of sample analysis. + represents certain positive identification, ~ represents uncertain, - represents certain negative identification. Numbers represent counts of patients with Raman detected crystals in this category (i.e. gout, MSU+ CPLM had 1 patient with calcite crystals).
Results of sample analysis. + represents certain positive identification, ~ represents uncertain, - represents certain negative identification. Numbers represent counts of patients with Raman detected crystals in this category (i.e. gout, MSU+ CPLM had 1 patient with calcite crystals).
Disclosures: T. Niessink, None; M. Janssen, None; C. Otto, Hybriscan BV; T. Jansen, None.
Background/Purpose: The current golden standard in diagnosing gout and calcium pyrophosphate deposition is polarized light microscopy (PLM). However, small crystal sizes, the presence of other crystal types, glass artefacts and cellular uptake of MSU can pose uncertainties in the clinical analysis of synovial fluids. There are multiple methods to improve synovial fluid analysis, of which Raman spectroscopy is the most promising. In our approach, we have integrated an ordinary polarized light microscope with a Raman spectroscope (iRPolM, Hybriscan), allowing for simultaneous use of both techniques. In this abstract, we demonstrate the added value of this technique for the first 50 patients, including 23 diagnosed with gout.
Methods: 50 patients were punctured for diagnostic purposes when presenting with swollen joints. Patient characteristics are given in figure 1. Samples were analyzed by an experienced rheumatologist with polarization microscopy, who also gave an indication of certainty in diagnosis. The iRPolM analysis followed this. A visual representation of this method can be found in figure 2. Retrieved Raman spectra were analyzed by a trained Raman spectroscopist for the presence of MSU, calcium pyrophosphate dihydrate (CPPD) or other (deposited) materials.
Results: A summary of the results from this experiment can be found in figure 3. We demonstrate a high correspondence (95%) between a certain identification with PLM and iRPolM. While the rheumatologist is only able to identify MSU/CPPD, Raman can also discriminate other microparticles.
Hydroxyapatite crystals were found in 1 patient with RA and one patient with a degenerative shoulder syndrome. Calcite (calcium carbonate) could be identified in 17 patients, 4 of whom were diagnosed with gout, 4 with CPPD, 3 with Rheumatoid Arthritis, 2 with Osteoarthritis and 4 undefined.
Titanium dioxide crystals were found in 6 patients. Only one of 6 patients with titanium crystals had an orthopedic implant, there is no clear relation between the two.
Carotenoids, a precursor molecule for vitamin A, could be found in 4 patients, 1 of which was diagnosed with gout and 3 had an undefined joint disease. All thenardite (sodium sulphate) crystals could be found in patients with osteoarthritis. Microplastics could be found in 19 patients, but environmental pollution must be excluded.
Conclusion: Raman spectroscopy has a significant added value to PLM, as it can not only can discriminate between MSU and CPPD but also identify a whole range of other crystals which might be related to potentially novel crystallopathies. In the future crystal identification in rheumatology may well have to rely on Raman based diagnostics.
 Patient characteristics table. * CPDD: calcium pyrophosphate deposition disease ** Undefined means joint disease other than gout, CPPD, rheumatoid arthritis or osteoarthritis. *** Yes/no means a 100% certain crystal identification as assessed by an experienced rheumatologist. Doubtful means a less than 100% certain crystal identification
Patient characteristics table. * CPDD: calcium pyrophosphate deposition disease ** Undefined means joint disease other than gout, CPPD, rheumatoid arthritis or osteoarthritis. *** Yes/no means a 100% certain crystal identification as assessed by an experienced rheumatologist. Doubtful means a less than 100% certain crystal identification Graphic depiction of the Raman analysis process. After scanning, Raman spectra are analyzed with visual examination by a trained spectroscopist. The presence of a peak at 630 wavenumber (cm-1) is specific for MSU crystals, whereas a peak at 1050 wavenumber (cm-1) is specific for calcium pyrophosphate crystals.
Graphic depiction of the Raman analysis process. After scanning, Raman spectra are analyzed with visual examination by a trained spectroscopist. The presence of a peak at 630 wavenumber (cm-1) is specific for MSU crystals, whereas a peak at 1050 wavenumber (cm-1) is specific for calcium pyrophosphate crystals.  Results of sample analysis. + represents certain positive identification, ~ represents uncertain, - represents certain negative identification. Numbers represent counts of patients with Raman detected crystals in this category (i.e. gout, MSU+ CPLM had 1 patient with calcite crystals).
Results of sample analysis. + represents certain positive identification, ~ represents uncertain, - represents certain negative identification. Numbers represent counts of patients with Raman detected crystals in this category (i.e. gout, MSU+ CPLM had 1 patient with calcite crystals).Disclosures: T. Niessink, None; M. Janssen, None; C. Otto, Hybriscan BV; T. Jansen, None.

