Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1762: Stability of Cell Bound Complement Activation Products (CB-CAPs), Multianalyte Assay Panel (MAP) with Algorithm, and Other Autoimmune Biomarkers Among Clinical Patients Throughout the SARS-CoV-2 Pandemic and Vaccination Campaigns
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MR
Mark Rudolph, PhD
Exagen, Inc
Vista, CA, United States
Abstract Poster Presenter(s)
Mark Rudolph, Rory Bloch, Anja Kammesheidt and Roberta Alexander, Exagen, Inc., Vista, CA
Background/Purpose: COVID-19 can cause autoantibody signatures in severely ill patients, and widespread emerging post-acute sequelae of SARS-CoV-2 (PASC) share many symptoms consistent with rheumatologic involvement (medRxiv 2021.09.21.21263845). Any impact on diagnosis or monitoring of rheumatological diseases has yet to be determined with a retrospective review of clinical laboratory results. This study investigated the potential impact of COVID-19 surges on autoimmune connective-tissue disease biomarkers. We measured a clinical diagnostic multianalyte assay panel (MAP), which includes cell-bound complement activation products (CB-CAPs) and numerous auto-antibodies, in a large number of samples collected throughout the United States.
Methods: Clinical autoimmune diagnostic tests were performed in Exagen Inc's clinical laboratory. Data were assembled from between the summer of 2019 and November 2021 and were grouped by United States census region to align any changes in biomarkers levels with well described COVID-19 waves (e.g. Winter 2020-21). Pre-COVID and COVID-era positivity rates were interrogated on individual MAP components (B-cell and erythrocyte CB-CAPs (BC4d and EC4d, respectively), anti-nuclear antibodies (ANA), anti-double-stranded DNA (dsDNA) anti-Smith antibodies, and several specificity components), the entire MAP, and other auto-antibody tests. F-tests were performed to describe variance, and p-values were determined by two-tailed t-test.
Results: In all, over 250,000 CB-CAP and MAP determinations were analyzed; approximately 37.5% of the results collected before March 2020, and the remaining 62.5% collected between March 2020 and November 2021. BC4d and EC4d showed no significant difference between pre-COVID-19 and COVID-19-era positivity rates (Figure 1a and b). MAP positivity also did not significantly change through the pandemic (Figure 1c). Two autoantibodies previously described in severe COVID-19 patients, anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM, show population-wide increases in positivity in late summer (for anti-B2GP IgG) and late fall (anti-RF IgM), concurrent with the increase in transmission that occurred during the 2nd and 3rd wave of virus (Figure 2).
Conclusion: We have shown that CB-CAPs positivity was stable pre- and post-SARS-CoV-2 vaccination rollout (Rudolph et al., ACR 2021). Here, we evaluated whether the COVID-19 pandemic influenced CB-CAPs, MAP, or autoantibody positivity. While we do not know how many patients have had COVID-19, or the vaccine, given the incidence of the SARS-CoV-2 virus and the large population for whom the MAP test was ordered, there is likely significant overlap between groups. As such, were infection to COVID-19 to lead to elevated CB-CAPs, we would expect an increase to be reflected in our data. However, CB-CAPs and MAP positivity rate has remained similar prior to and throughout the pandemic. Some autoantibodies did show increased positivity during the large 3rd wave (Nov 2020 – Feb 2021) of the COVID-19 pandemic in agreement with serology described in acute and some PASC patients. However, attributing these changes to COVID-19 infection would require a chart review undertaking.
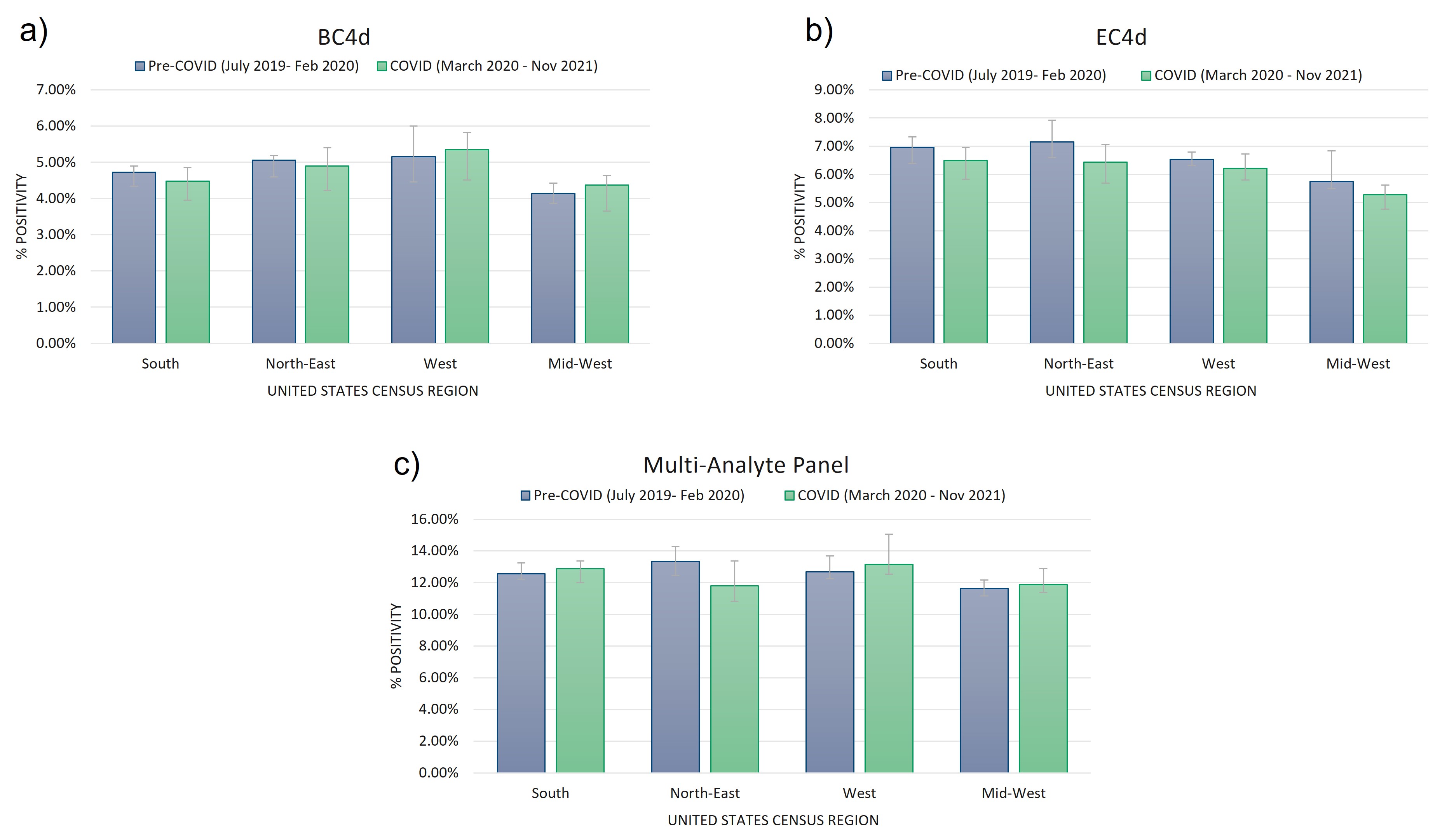 Figure 1) Cell-bound complement activation product (CB-CAPs) and multi-analyte panel (MAP) positivity rates are not impacted by the COVID-19 pandemic. Positivity rates of B-cell (a) and erythrocyte- (b) bound C4d (measured by flow cytometry, positive results defined by net MFI > 14 for EC4d, and net MFI > 60 for BC4d) was not significantly impacted by the COVID-19 pandemic among rheumatology patients tested at our central laboratory. Positivity rates of our mutli-analyte panel (MAP, described in Putterman C, et al. Lupus Science & Medicine. 2014) was also not significantly impacted by the COVID-19 pandemic among this cohort (c). Significance was defined by a p-value < 0.01 using two-tailed t-test (homoscedastic for equal variance, heteroscedastic for unequal variance).
Figure 1) Cell-bound complement activation product (CB-CAPs) and multi-analyte panel (MAP) positivity rates are not impacted by the COVID-19 pandemic. Positivity rates of B-cell (a) and erythrocyte- (b) bound C4d (measured by flow cytometry, positive results defined by net MFI > 14 for EC4d, and net MFI > 60 for BC4d) was not significantly impacted by the COVID-19 pandemic among rheumatology patients tested at our central laboratory. Positivity rates of our mutli-analyte panel (MAP, described in Putterman C, et al. Lupus Science & Medicine. 2014) was also not significantly impacted by the COVID-19 pandemic among this cohort (c). Significance was defined by a p-value < 0.01 using two-tailed t-test (homoscedastic for equal variance, heteroscedastic for unequal variance).
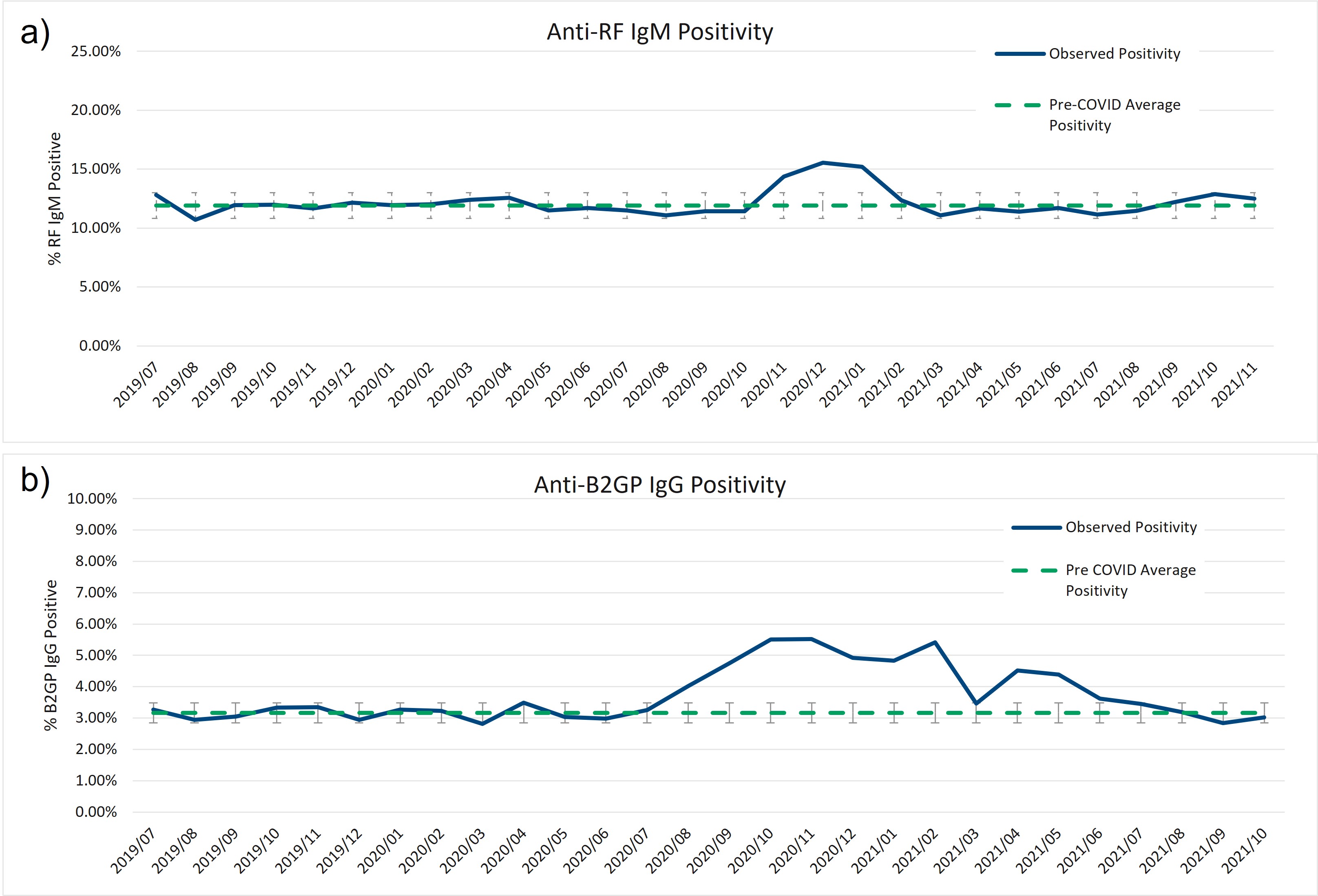 Figure 2) Increase in anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM positivity between late-2020 and early-2021. Anti-rheumatoid factor (RF) IgM and anti-beta-2-glycoprotein (B2GP) IgG show increases of greater than 2 standard deviations over the pre-COVID assay positivity mean centered around the large 3rd COVID wave towards the end of 2020.
Figure 2) Increase in anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM positivity between late-2020 and early-2021. Anti-rheumatoid factor (RF) IgM and anti-beta-2-glycoprotein (B2GP) IgG show increases of greater than 2 standard deviations over the pre-COVID assay positivity mean centered around the large 3rd COVID wave towards the end of 2020.
Disclosures: M. Rudolph, Exagen, Inc; R. Bloch, Exagen; A. Kammesheidt, Exagen Inc.; R. Alexander, Exagen Inc..
Background/Purpose: COVID-19 can cause autoantibody signatures in severely ill patients, and widespread emerging post-acute sequelae of SARS-CoV-2 (PASC) share many symptoms consistent with rheumatologic involvement (medRxiv 2021.09.21.21263845). Any impact on diagnosis or monitoring of rheumatological diseases has yet to be determined with a retrospective review of clinical laboratory results. This study investigated the potential impact of COVID-19 surges on autoimmune connective-tissue disease biomarkers. We measured a clinical diagnostic multianalyte assay panel (MAP), which includes cell-bound complement activation products (CB-CAPs) and numerous auto-antibodies, in a large number of samples collected throughout the United States.
Methods: Clinical autoimmune diagnostic tests were performed in Exagen Inc's clinical laboratory. Data were assembled from between the summer of 2019 and November 2021 and were grouped by United States census region to align any changes in biomarkers levels with well described COVID-19 waves (e.g. Winter 2020-21). Pre-COVID and COVID-era positivity rates were interrogated on individual MAP components (B-cell and erythrocyte CB-CAPs (BC4d and EC4d, respectively), anti-nuclear antibodies (ANA), anti-double-stranded DNA (dsDNA) anti-Smith antibodies, and several specificity components), the entire MAP, and other auto-antibody tests. F-tests were performed to describe variance, and p-values were determined by two-tailed t-test.
Results: In all, over 250,000 CB-CAP and MAP determinations were analyzed; approximately 37.5% of the results collected before March 2020, and the remaining 62.5% collected between March 2020 and November 2021. BC4d and EC4d showed no significant difference between pre-COVID-19 and COVID-19-era positivity rates (Figure 1a and b). MAP positivity also did not significantly change through the pandemic (Figure 1c). Two autoantibodies previously described in severe COVID-19 patients, anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM, show population-wide increases in positivity in late summer (for anti-B2GP IgG) and late fall (anti-RF IgM), concurrent with the increase in transmission that occurred during the 2nd and 3rd wave of virus (Figure 2).
Conclusion: We have shown that CB-CAPs positivity was stable pre- and post-SARS-CoV-2 vaccination rollout (Rudolph et al., ACR 2021). Here, we evaluated whether the COVID-19 pandemic influenced CB-CAPs, MAP, or autoantibody positivity. While we do not know how many patients have had COVID-19, or the vaccine, given the incidence of the SARS-CoV-2 virus and the large population for whom the MAP test was ordered, there is likely significant overlap between groups. As such, were infection to COVID-19 to lead to elevated CB-CAPs, we would expect an increase to be reflected in our data. However, CB-CAPs and MAP positivity rate has remained similar prior to and throughout the pandemic. Some autoantibodies did show increased positivity during the large 3rd wave (Nov 2020 – Feb 2021) of the COVID-19 pandemic in agreement with serology described in acute and some PASC patients. However, attributing these changes to COVID-19 infection would require a chart review undertaking.
 Figure 1) Cell-bound complement activation product (CB-CAPs) and multi-analyte panel (MAP) positivity rates are not impacted by the COVID-19 pandemic. Positivity rates of B-cell (a) and erythrocyte- (b) bound C4d (measured by flow cytometry, positive results defined by net MFI > 14 for EC4d, and net MFI > 60 for BC4d) was not significantly impacted by the COVID-19 pandemic among rheumatology patients tested at our central laboratory. Positivity rates of our mutli-analyte panel (MAP, described in Putterman C, et al. Lupus Science & Medicine. 2014) was also not significantly impacted by the COVID-19 pandemic among this cohort (c). Significance was defined by a p-value < 0.01 using two-tailed t-test (homoscedastic for equal variance, heteroscedastic for unequal variance).
Figure 1) Cell-bound complement activation product (CB-CAPs) and multi-analyte panel (MAP) positivity rates are not impacted by the COVID-19 pandemic. Positivity rates of B-cell (a) and erythrocyte- (b) bound C4d (measured by flow cytometry, positive results defined by net MFI > 14 for EC4d, and net MFI > 60 for BC4d) was not significantly impacted by the COVID-19 pandemic among rheumatology patients tested at our central laboratory. Positivity rates of our mutli-analyte panel (MAP, described in Putterman C, et al. Lupus Science & Medicine. 2014) was also not significantly impacted by the COVID-19 pandemic among this cohort (c). Significance was defined by a p-value < 0.01 using two-tailed t-test (homoscedastic for equal variance, heteroscedastic for unequal variance). Figure 2) Increase in anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM positivity between late-2020 and early-2021. Anti-rheumatoid factor (RF) IgM and anti-beta-2-glycoprotein (B2GP) IgG show increases of greater than 2 standard deviations over the pre-COVID assay positivity mean centered around the large 3rd COVID wave towards the end of 2020.
Figure 2) Increase in anti-beta-2-glycoprotein IgG and anti-rheumatoid factor IgM positivity between late-2020 and early-2021. Anti-rheumatoid factor (RF) IgM and anti-beta-2-glycoprotein (B2GP) IgG show increases of greater than 2 standard deviations over the pre-COVID assay positivity mean centered around the large 3rd COVID wave towards the end of 2020. Disclosures: M. Rudolph, Exagen, Inc; R. Bloch, Exagen; A. Kammesheidt, Exagen Inc.; R. Alexander, Exagen Inc..

