Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1761: Treatment Patterns of Disease-Modifying Anti-Rheumatic Drugs by Serostatus Among Patients with Rheumatoid Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YJ
Yinzhu Jin, MS
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Yinzhu Jin, Jun Liu, Rishi Desai and Seoyoung Kim, Brigham and Women's Hospital, Boston, MA
Background/Purpose: Previous studies suggest that seropositive and seronegative rheumatoid arthritis (RA) patients may respond differently to disease-modifying anti-rheumatic drugs (DMARDs). However, little is known about variation in treatment strategies between seropositive and seronegative RA patients in routine care. We aimed to examine the patterns of DMARD treatment among RA by serostatus in a real-world setting.
Methods: Using Optum Clinformatics® Data Mart (01/01/2004 - 03/31/2021) that includes results for outpatient laboratory tests, we identified new users of conventional synthetic DMARDs (csDMARDs), tumor necrosis factor-alpha inhibitors (TNFi, i.e., adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), abatacept, interleukin (IL)-6 inhibitors (i.e., sarilumab, tocilizumab), and Janus kinase inhibitors (JAKi, i.e., tofacitinib). Dispensing of the first-ever DMARD was defined as the index date. TNFi, abatacept, IL-6 inhibitor, and JAKi users were allowed to have prior csDMARDs. Eligible subjects were required to 1) have ≥2 RA diagnostic codes separated by 7-365 days before the index date, 2) have ≥365 days of continuous enrollment within 1 year before and after the index date, and 3) be ≥18 years old. To assess the serostatus, we further restricted to patients who had rheumatoid factor (RF) or anticitrullinated protein antibody (ACPA) results or International Classification of Diseases Tenth Version (ICD-10) code of M05*/M06.0* any time prior to the index date. The primary outcome of interest was the treatment patterns during 1-year after the initiation of DMARD. If a patient continuously used the index DMARD (with a 60-day gap) and did not receive any other DMARDs, the patient was defined as "persistent user". "Non-persistent users" were those who discontinued their index DMARD before the end of follow-up. "Switchers" were defined as those having initiated other biologics or JAKi during the follow-up. A multivariable logistic regression model was conducted to estimate the adjusted odds ratio (aOR) of treatment persistence vs. discontinuation/switching for seropositive compared to seronegative patients.
Results: We identified a total of 35429 RA patients: 60% seropositive and 40% seronegative. There were 65.3% csDMARD, 28.2% TNFi, 2.8% abatacept, 1.2% IL-6 inhibitor, and 2.5% JAK inhibitor initiators (Table). The mean age was 60 years old, and 75.9% were female. Within each DMARD group, seropositive patients were 57% in csDMARD, 62.7% in TNFi, 71.0% in abatacept, 74.4% in IL-6 inhibitor, and 80.1% in JAKi. Seropositive patients had a slightly higher proportion of persistence compared to seronegative patients (Figure 1). After adjusting for potential confounders, we observed aOR of 1.11 (95% CI: 1.01, 1.21) of treatment persistence for seropositive compared to seronegative patients (Figure 2).
Conclusion: Among RA patients who newly initiated a DMARD, seropositive patients had a modest increase in treatment persistence compared to seronegative patients. Further studies are necessary to examine whether the clinical effectiveness of different DMARDs varies based on seropositivity.
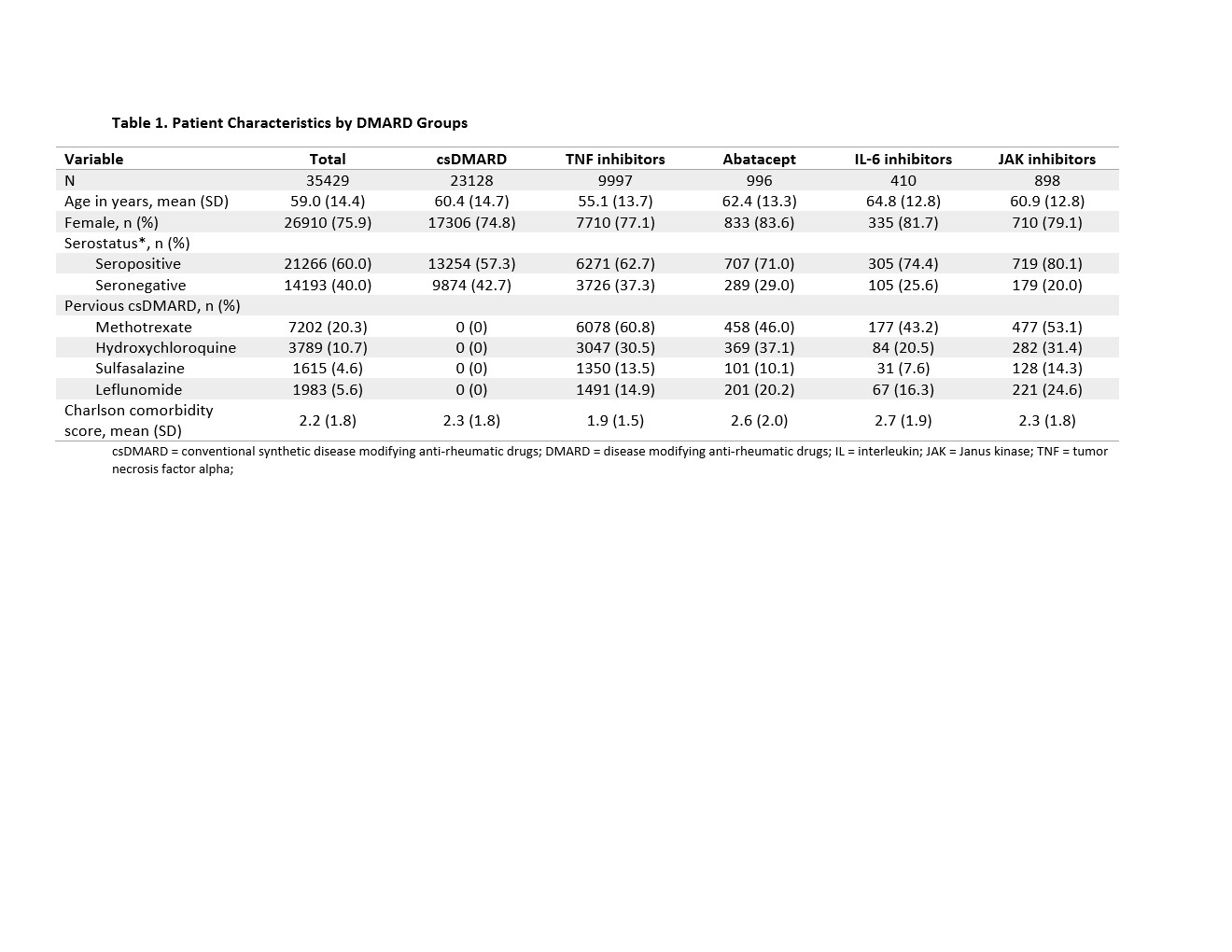 Table 1. Patient Characteristics by DMARD Groups
Table 1. Patient Characteristics by DMARD Groups
csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha;
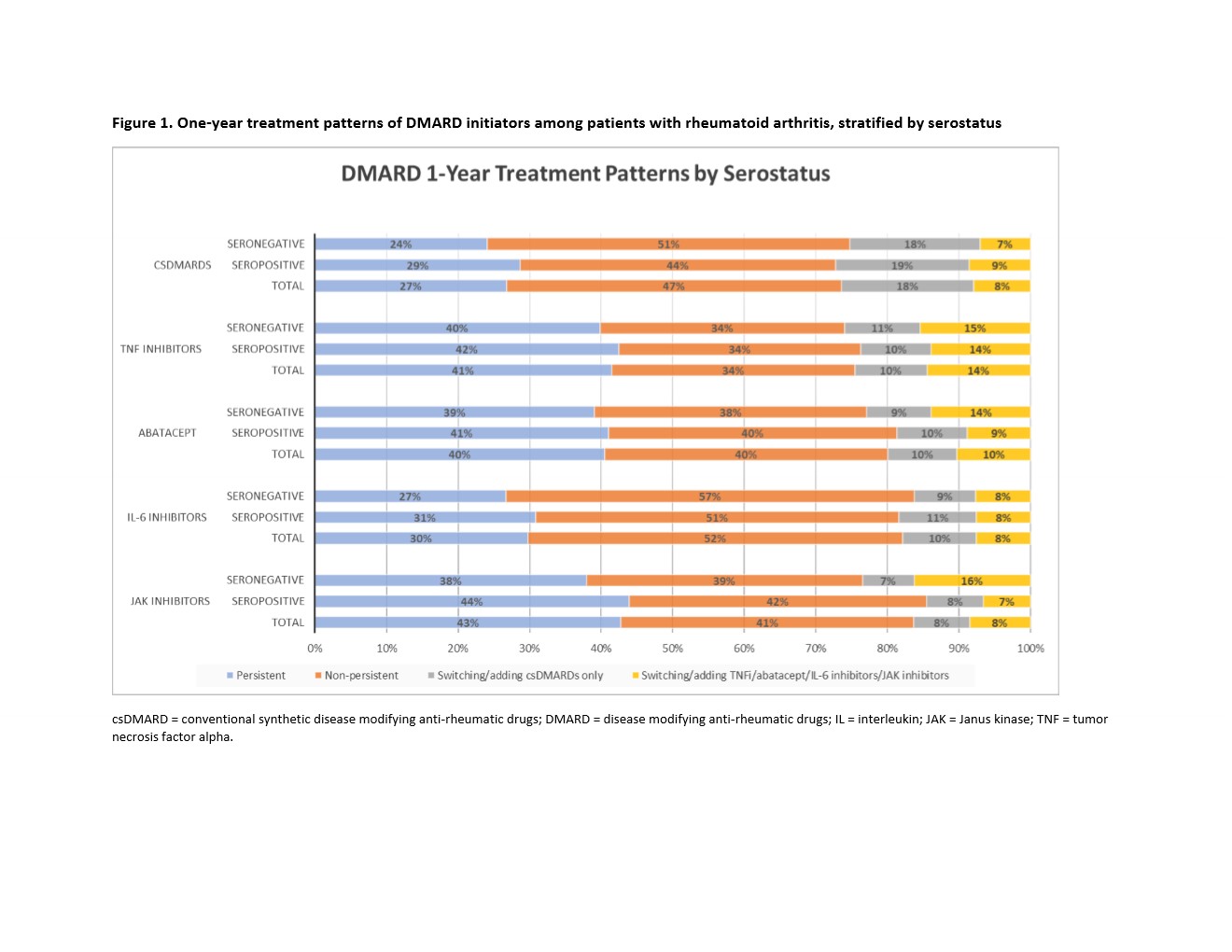 Figure 1. One-year treatment patterns of DMARD initiators among patients with rheumatoid arthritis, stratified by serostatus
Figure 1. One-year treatment patterns of DMARD initiators among patients with rheumatoid arthritis, stratified by serostatus
csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha.
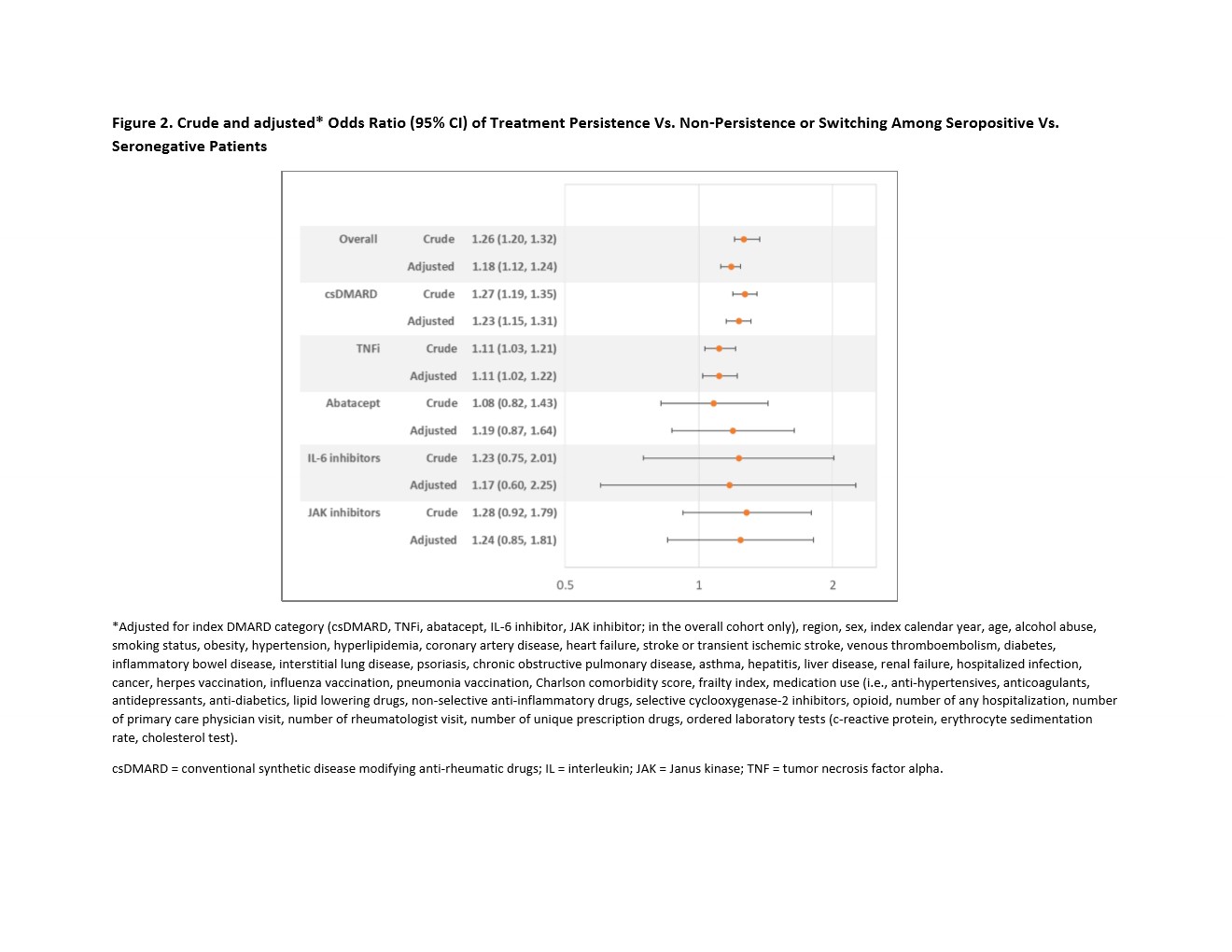 Figure 2. Crude and adjusted* Odds Ratio (95% CI) of Treatment Persistence Vs. Non-Persistence or Switching Among Seropositive Vs. Seronegative Patients
Figure 2. Crude and adjusted* Odds Ratio (95% CI) of Treatment Persistence Vs. Non-Persistence or Switching Among Seropositive Vs. Seronegative Patients
*Adjusted for index DMARD category (csDMARD, TNFi, abatacept, IL-6 inhibitor, JAK inhibitor; in the overall cohort only), region, sex, index calendar year, age, alcohol abuse, smoking status, obesity, hypertension, hyperlipidemia, coronary artery disease, heart failure, stroke or transient ischemic stroke, venous thromboembolism, diabetes, inflammatory bowel disease, interstitial lung disease, psoriasis, chronic obstructive pulmonary disease, asthma, hepatitis, liver disease, renal failure, hospitalized infection, cancer, herpes vaccination, influenza vaccination, pneumonia vaccination, Charlson comorbidity score, frailty index, medication use (i.e., anti-hypertensives, anticoagulants, antidepressants, anti-diabetics, lipid lowering drugs, non-selective anti-inflammatory drugs, selective cyclooxygenase-2 inhibitors, opioid, number of any hospitalization, number of primary care physician visit, number of rheumatologist visit, number of unique prescription drugs, ordered laboratory tests (c-reactive protein, erythrocyte sedimentation rate, cholesterol test).
csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha.
Disclosures: Y. Jin, None; J. Liu, None; R. Desai, Bayer, Vertex, Novartis; S. Kim, Pfizer, AbbVie/Abbott, Roche, Bristol-Myers Squibb(BMS).
Background/Purpose: Previous studies suggest that seropositive and seronegative rheumatoid arthritis (RA) patients may respond differently to disease-modifying anti-rheumatic drugs (DMARDs). However, little is known about variation in treatment strategies between seropositive and seronegative RA patients in routine care. We aimed to examine the patterns of DMARD treatment among RA by serostatus in a real-world setting.
Methods: Using Optum Clinformatics® Data Mart (01/01/2004 - 03/31/2021) that includes results for outpatient laboratory tests, we identified new users of conventional synthetic DMARDs (csDMARDs), tumor necrosis factor-alpha inhibitors (TNFi, i.e., adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), abatacept, interleukin (IL)-6 inhibitors (i.e., sarilumab, tocilizumab), and Janus kinase inhibitors (JAKi, i.e., tofacitinib). Dispensing of the first-ever DMARD was defined as the index date. TNFi, abatacept, IL-6 inhibitor, and JAKi users were allowed to have prior csDMARDs. Eligible subjects were required to 1) have ≥2 RA diagnostic codes separated by 7-365 days before the index date, 2) have ≥365 days of continuous enrollment within 1 year before and after the index date, and 3) be ≥18 years old. To assess the serostatus, we further restricted to patients who had rheumatoid factor (RF) or anticitrullinated protein antibody (ACPA) results or International Classification of Diseases Tenth Version (ICD-10) code of M05*/M06.0* any time prior to the index date. The primary outcome of interest was the treatment patterns during 1-year after the initiation of DMARD. If a patient continuously used the index DMARD (with a 60-day gap) and did not receive any other DMARDs, the patient was defined as "persistent user". "Non-persistent users" were those who discontinued their index DMARD before the end of follow-up. "Switchers" were defined as those having initiated other biologics or JAKi during the follow-up. A multivariable logistic regression model was conducted to estimate the adjusted odds ratio (aOR) of treatment persistence vs. discontinuation/switching for seropositive compared to seronegative patients.
Results: We identified a total of 35429 RA patients: 60% seropositive and 40% seronegative. There were 65.3% csDMARD, 28.2% TNFi, 2.8% abatacept, 1.2% IL-6 inhibitor, and 2.5% JAK inhibitor initiators (Table). The mean age was 60 years old, and 75.9% were female. Within each DMARD group, seropositive patients were 57% in csDMARD, 62.7% in TNFi, 71.0% in abatacept, 74.4% in IL-6 inhibitor, and 80.1% in JAKi. Seropositive patients had a slightly higher proportion of persistence compared to seronegative patients (Figure 1). After adjusting for potential confounders, we observed aOR of 1.11 (95% CI: 1.01, 1.21) of treatment persistence for seropositive compared to seronegative patients (Figure 2).
Conclusion: Among RA patients who newly initiated a DMARD, seropositive patients had a modest increase in treatment persistence compared to seronegative patients. Further studies are necessary to examine whether the clinical effectiveness of different DMARDs varies based on seropositivity.
 Table 1. Patient Characteristics by DMARD Groups
Table 1. Patient Characteristics by DMARD GroupscsDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha;
 Figure 1. One-year treatment patterns of DMARD initiators among patients with rheumatoid arthritis, stratified by serostatus
Figure 1. One-year treatment patterns of DMARD initiators among patients with rheumatoid arthritis, stratified by serostatuscsDMARD = conventional synthetic disease modifying anti-rheumatic drugs; DMARD = disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha.
 Figure 2. Crude and adjusted* Odds Ratio (95% CI) of Treatment Persistence Vs. Non-Persistence or Switching Among Seropositive Vs. Seronegative Patients
Figure 2. Crude and adjusted* Odds Ratio (95% CI) of Treatment Persistence Vs. Non-Persistence or Switching Among Seropositive Vs. Seronegative Patients*Adjusted for index DMARD category (csDMARD, TNFi, abatacept, IL-6 inhibitor, JAK inhibitor; in the overall cohort only), region, sex, index calendar year, age, alcohol abuse, smoking status, obesity, hypertension, hyperlipidemia, coronary artery disease, heart failure, stroke or transient ischemic stroke, venous thromboembolism, diabetes, inflammatory bowel disease, interstitial lung disease, psoriasis, chronic obstructive pulmonary disease, asthma, hepatitis, liver disease, renal failure, hospitalized infection, cancer, herpes vaccination, influenza vaccination, pneumonia vaccination, Charlson comorbidity score, frailty index, medication use (i.e., anti-hypertensives, anticoagulants, antidepressants, anti-diabetics, lipid lowering drugs, non-selective anti-inflammatory drugs, selective cyclooxygenase-2 inhibitors, opioid, number of any hospitalization, number of primary care physician visit, number of rheumatologist visit, number of unique prescription drugs, ordered laboratory tests (c-reactive protein, erythrocyte sedimentation rate, cholesterol test).
csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; IL = interleukin; JAK = Janus kinase; TNF = tumor necrosis factor alpha.
Disclosures: Y. Jin, None; J. Liu, None; R. Desai, Bayer, Vertex, Novartis; S. Kim, Pfizer, AbbVie/Abbott, Roche, Bristol-Myers Squibb(BMS).

