Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0599: Expansion of HLA-DR+CD45RAhi Non-lymphoid Cells in Patients with Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CG
Christian Geier, MD
SUNY Upstate Medical University
Syracuse, NY, United States
Abstract Poster Presenter(s)
Christian Geier1, Andras Perl2 and Robert Winchester3, 1SUNY Upstate Medical University, Syracuse, NY, 2SUNY, Syracuse, NY, 3Columbia University, New York, NY
Background/Purpose: In RA, aberrant lymphocytes can damage synovial joints and other organs. Antigen-presenting cells (APC) can activate lymphocytes and are considered critical to initiate immune responses; we reason that APC may have relevance for the pathologic auto-immune response in RA. Surprisingly, we previously found a striking depletion of two potent APC lineages — CD141+ and CD1c+ myeloid dendritic cells — in RA. More interestingly, this was accompanied by the emergence of previously undetected cells (expressing unorthodox marker combinations, precluding their categorization into existing definitions) with APC potential (DR+). These cells expressed CD45RA, leading us to hypothesize that the inclusion of this isoform can help categorize myeloid APC more adequately.
Methods: RA patients satisfying the 2010 ACR classification criteria and matched healthy donors (n=10 each), underwent spectral cytometry to characterize myeloid APC in peripheral blood mononuclear cells. We gated myeloid APC based on forward and side scatter, HLA-DR expression and lack of markers for established monocytic lineages. We removed lymphocytes and non-viable cells using a CD3/CD19 dump and an amine-reactive viability dye. Plasmacytoid DC were excluded using CD303, resulting in a population containing established myeloid DC while retaining potentially new phenotypes; we refer to this definition as 'candidate APC' We then categorized candidate APC based on expression of CD45RA and CD1c. We calculated median fluorescence intensities of co-stimulatory/inhibitory molecules, and chemokine receptors. Kruskal-Wallis testing with a threshold of p< 0.05 was performed to assess for significant differences.
Results: Healthy donor APCs were dominated by CD1c+CD45RAint cells, consistent with established myeloid dendritic cells whereas in RA — as expected — myeloid dendritic cells were markedly decreased. (p = 0.019). Interestingly, we found that in RA two additional subsets, both characterized by much higher CD45RA expression, were significantly increased: CD45RAhi CD1c+ (p = 0.028) and CD45hiCD1c- (p = 0.049). In RA, the CD45RAhi subsets differed significantly in their expression of co-stimulatory/inhibitory and chemokine receptors: enhanced expression of CD86 in CD45RAhi CD1c- (p = 0.049). Both CD45RAhi populations showed dramatically higher expression of CD197 (CCR7; p < 0.01) compared with CD45RAint myeloid DC while exhibiting a lower expression of CD155 (poliovirus receptor; p < 0.01). CD83 and CD275 (ICOS-L) expression did not differ.
Conclusion: The blood of RA patients contains HLA-DR+ cells that do not fit existing definitions. These potential APC can be effectively captured by gating all DR+ cells which, with the addition of CD45RA and CD1c, can be categorized into subsets that suggest migratory and co-stimulatory capacity distinct from established CD1c+CD45RAint DC. A higher expression of CCR7, and in the case of the CD1c- subset, CD86, shows the potential for these putative APC to migrate to lymph nodes and, via CD28, provide pro-inflammatory stimulation to T lymphocytes.
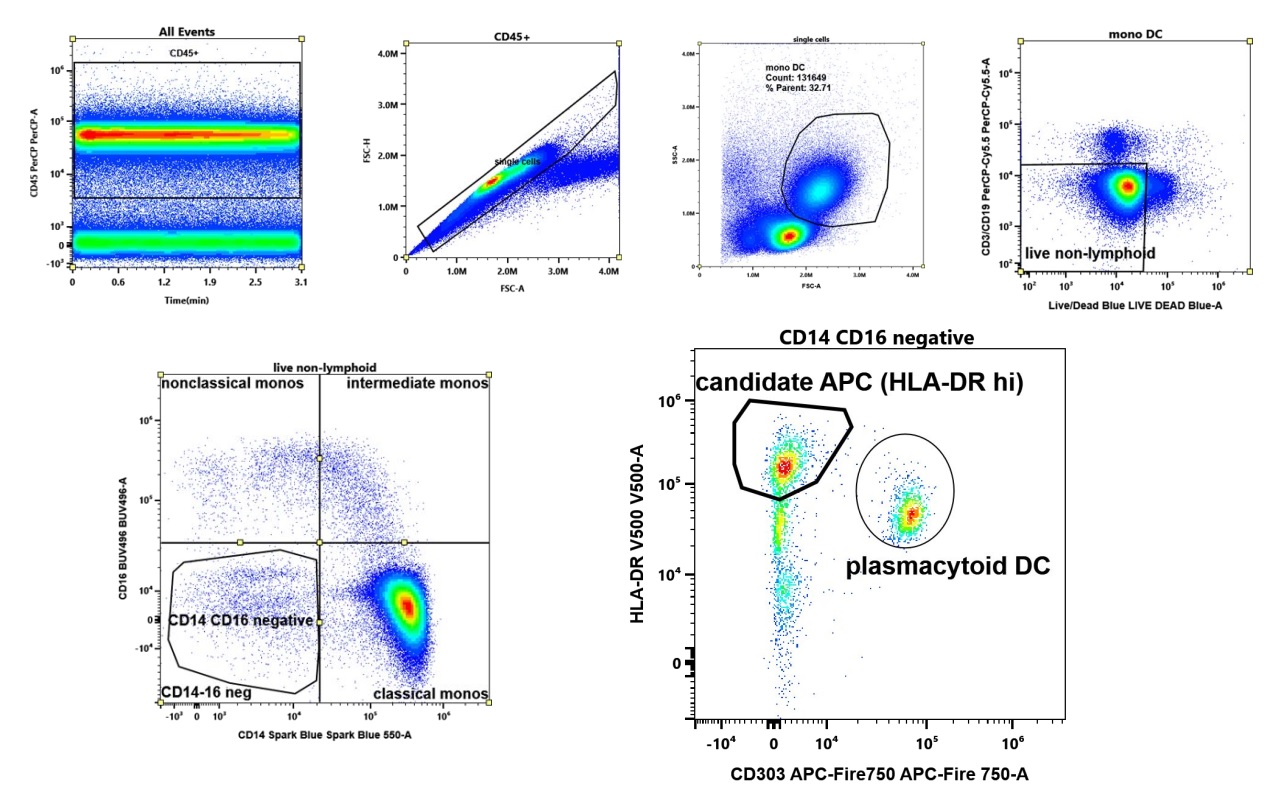 Gating strategy to capture candidate APC. We include all viable HLA-DR expressing cells lacking expression of lymphoid (CD3,CD19), monocytic markers (CD14, CD16), and plasmacytoid dendritic cells (CD303).
Gating strategy to capture candidate APC. We include all viable HLA-DR expressing cells lacking expression of lymphoid (CD3,CD19), monocytic markers (CD14, CD16), and plasmacytoid dendritic cells (CD303).
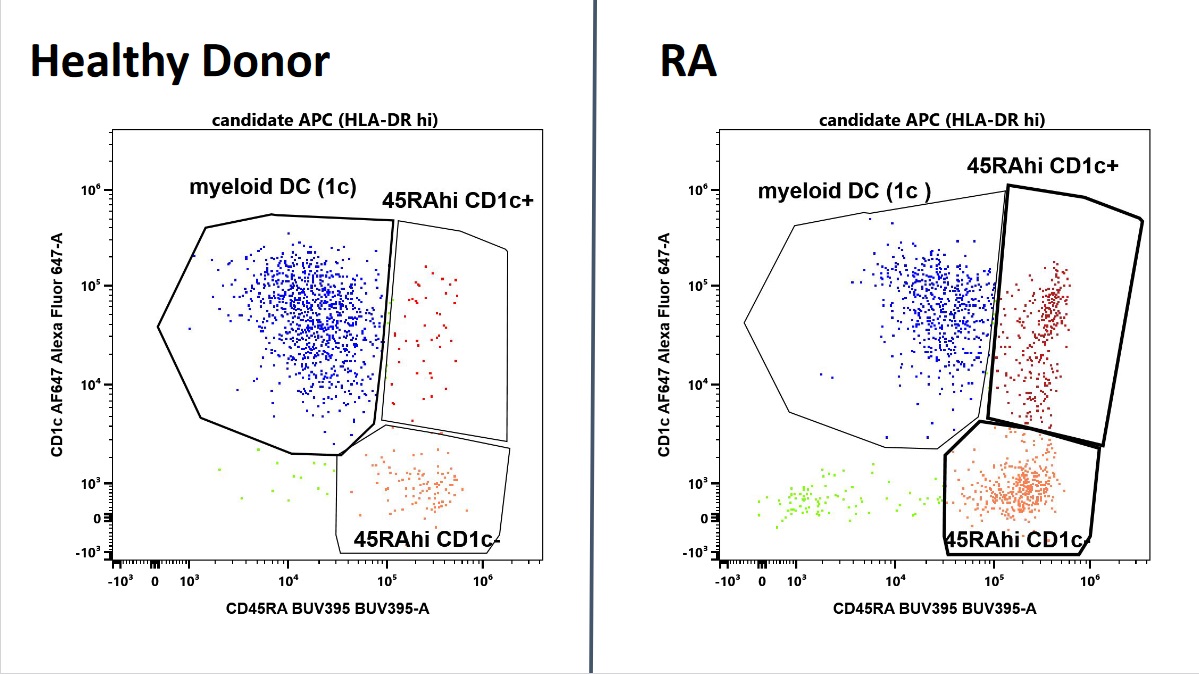 Characterization of candidate APC by CD45RA and CD1c expression. In blue, CD45intCD1c positive cells, consistent with myeloid dendritic cells, dominate in health and are decreased in RA . In contrast, two CD45RAhi populations are expanded (red). Shown are representative plots of an RA patient and a healthy donor of similar age and matching age and ethnicity.
Characterization of candidate APC by CD45RA and CD1c expression. In blue, CD45intCD1c positive cells, consistent with myeloid dendritic cells, dominate in health and are decreased in RA . In contrast, two CD45RAhi populations are expanded (red). Shown are representative plots of an RA patient and a healthy donor of similar age and matching age and ethnicity.
Disclosures: C. Geier, None; A. Perl, None; R. Winchester, None.
Background/Purpose: In RA, aberrant lymphocytes can damage synovial joints and other organs. Antigen-presenting cells (APC) can activate lymphocytes and are considered critical to initiate immune responses; we reason that APC may have relevance for the pathologic auto-immune response in RA. Surprisingly, we previously found a striking depletion of two potent APC lineages — CD141+ and CD1c+ myeloid dendritic cells — in RA. More interestingly, this was accompanied by the emergence of previously undetected cells (expressing unorthodox marker combinations, precluding their categorization into existing definitions) with APC potential (DR+). These cells expressed CD45RA, leading us to hypothesize that the inclusion of this isoform can help categorize myeloid APC more adequately.
Methods: RA patients satisfying the 2010 ACR classification criteria and matched healthy donors (n=10 each), underwent spectral cytometry to characterize myeloid APC in peripheral blood mononuclear cells. We gated myeloid APC based on forward and side scatter, HLA-DR expression and lack of markers for established monocytic lineages. We removed lymphocytes and non-viable cells using a CD3/CD19 dump and an amine-reactive viability dye. Plasmacytoid DC were excluded using CD303, resulting in a population containing established myeloid DC while retaining potentially new phenotypes; we refer to this definition as 'candidate APC' We then categorized candidate APC based on expression of CD45RA and CD1c. We calculated median fluorescence intensities of co-stimulatory/inhibitory molecules, and chemokine receptors. Kruskal-Wallis testing with a threshold of p< 0.05 was performed to assess for significant differences.
Results: Healthy donor APCs were dominated by CD1c+CD45RAint cells, consistent with established myeloid dendritic cells whereas in RA — as expected — myeloid dendritic cells were markedly decreased. (p = 0.019). Interestingly, we found that in RA two additional subsets, both characterized by much higher CD45RA expression, were significantly increased: CD45RAhi CD1c+ (p = 0.028) and CD45hiCD1c- (p = 0.049). In RA, the CD45RAhi subsets differed significantly in their expression of co-stimulatory/inhibitory and chemokine receptors: enhanced expression of CD86 in CD45RAhi CD1c- (p = 0.049). Both CD45RAhi populations showed dramatically higher expression of CD197 (CCR7; p < 0.01) compared with CD45RAint myeloid DC while exhibiting a lower expression of CD155 (poliovirus receptor; p < 0.01). CD83 and CD275 (ICOS-L) expression did not differ.
Conclusion: The blood of RA patients contains HLA-DR+ cells that do not fit existing definitions. These potential APC can be effectively captured by gating all DR+ cells which, with the addition of CD45RA and CD1c, can be categorized into subsets that suggest migratory and co-stimulatory capacity distinct from established CD1c+CD45RAint DC. A higher expression of CCR7, and in the case of the CD1c- subset, CD86, shows the potential for these putative APC to migrate to lymph nodes and, via CD28, provide pro-inflammatory stimulation to T lymphocytes.
 Gating strategy to capture candidate APC. We include all viable HLA-DR expressing cells lacking expression of lymphoid (CD3,CD19), monocytic markers (CD14, CD16), and plasmacytoid dendritic cells (CD303).
Gating strategy to capture candidate APC. We include all viable HLA-DR expressing cells lacking expression of lymphoid (CD3,CD19), monocytic markers (CD14, CD16), and plasmacytoid dendritic cells (CD303). Characterization of candidate APC by CD45RA and CD1c expression. In blue, CD45intCD1c positive cells, consistent with myeloid dendritic cells, dominate in health and are decreased in RA . In contrast, two CD45RAhi populations are expanded (red). Shown are representative plots of an RA patient and a healthy donor of similar age and matching age and ethnicity.
Characterization of candidate APC by CD45RA and CD1c expression. In blue, CD45intCD1c positive cells, consistent with myeloid dendritic cells, dominate in health and are decreased in RA . In contrast, two CD45RAhi populations are expanded (red). Shown are representative plots of an RA patient and a healthy donor of similar age and matching age and ethnicity. Disclosures: C. Geier, None; A. Perl, None; R. Winchester, None.

