Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0724–0751) Health Services Research Poster II
0743: Home-Based Telehealth in Rheumatology: A Systematic Review & Narrative Synthesis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AP
Alexander Peck, DO
Cedars-Sinai Medical Center
Los Angeles, CA, United States
Abstract Poster Presenter(s)
Alexander Peck1, Rebecca Grainger2, Jeffrey Curtis3, John Cush4, Neelkamal Soares5, Nikhil Davuluri6, William Benjamin Nowell7, Sandeep Sodhi8, Danielle Grauer8, Natalie Fortune6, Shilpa Venkatachalam9, Daniel Kirby10, Jeffrey Alper11, Kelly Gavigan12, Laura Stradford7, David Curtis12 and Swamy Venuturupalli1, 1Cedars-Sinai Medical Center, Los Angeles, CA, 2University of Otago, Wellington, New Zealand, 3Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 4RheumNow, Dallas, TX, 5Western Michigan University, Kalamazoo, MI, 6Attune Health, Beverly HIlls, CA, 7Global Healthy Living Foundation, Nyack, NY, 8Illumination Health, Birmingham, AL, 9Global Healthy Living Foundation, New York, NY, 10Bendcare, Charlotte, NC, 11Bendcare, Naples, 12Global Healthy Living Foundation, Upper Nyack, NY
Background/Purpose: Home based telehealth (HBT) visits, where a patient is located at home with a remote provider, became common during the COVID-19 pandemic. Patients now request HBT, even though in-person visits are possible. We conducted a systematic literature search with narrative synthesis on the use of HBT in rheumatology with the aim of informing the clinical practice of rheumatology using HBT.
Methods: We searched Cochrane, PubMed, and Web of Science databases using a pre-defined search strategy (Figure 1). A total of 2269 articles were retrieved and then subjected to further review (Figure 2). Two independent reviewers assessed whether the articles specifically addressed our objectives of determining HBT suitability, choosing telehealth-appropriate disease outcome measures, and identifying instruments suitable for measuring patient acceptance of HBT. Data was then extracted for a narrative summary and gap analysis.
Results: Seventeen studies and four abstracts met inclusion criteria and were included in the synthesis. Study heterogeneity precluded a meta-analysis. The key findings were as follows: Two studies support the use of pre-visit triage for new patients with inflammatory back pain and for early inflammatory arthritis by using telephone survey tools and enrolling Spondyloarthropathy patients for inflammatory back pain and rheumatologist-diagnosed inflammatory arthritis patients as control groups, respectively. Two survey studies reported providers had good acceptance of HBT for stable patients compared to more active patients or more complex presentations. Four studies reported successful use of remotely collected disease activity measures and PROs and reported concordance between PROs (RAID and RAPID3) with DAS-28 in low disease activity but not higher disease activity states. Another study supported the use of protocolized serum uric acid management in gout via telehealth visits. Two studies examined reported that telehealth satisfaction was generally high but was lower with increasing patient age.
Conclusion: A systematic literature search for articles addressing HBT in rheumatology provides useful, albeit limited, data as to how HBT can safely and effectively complement in-person care for certain rheumatologic conditions, particularly inflammatory arthritis, and gout. The data also provides some guidance on the appropriate selection of patients for telehealth. Further, the data highlights key gaps that future research should address such as creating strategies to identify patients whose health needs can be adequately addressed by HBT and optimizing methods to collect PROs and disease activity measures during HBT.
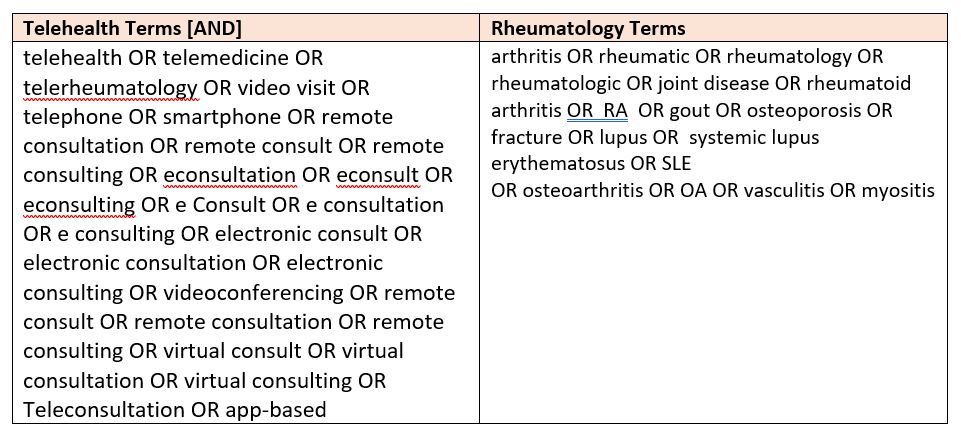 Figure 1: Search Strategy used to Retrieve Telehealth Articles
Figure 1: Search Strategy used to Retrieve Telehealth Articles
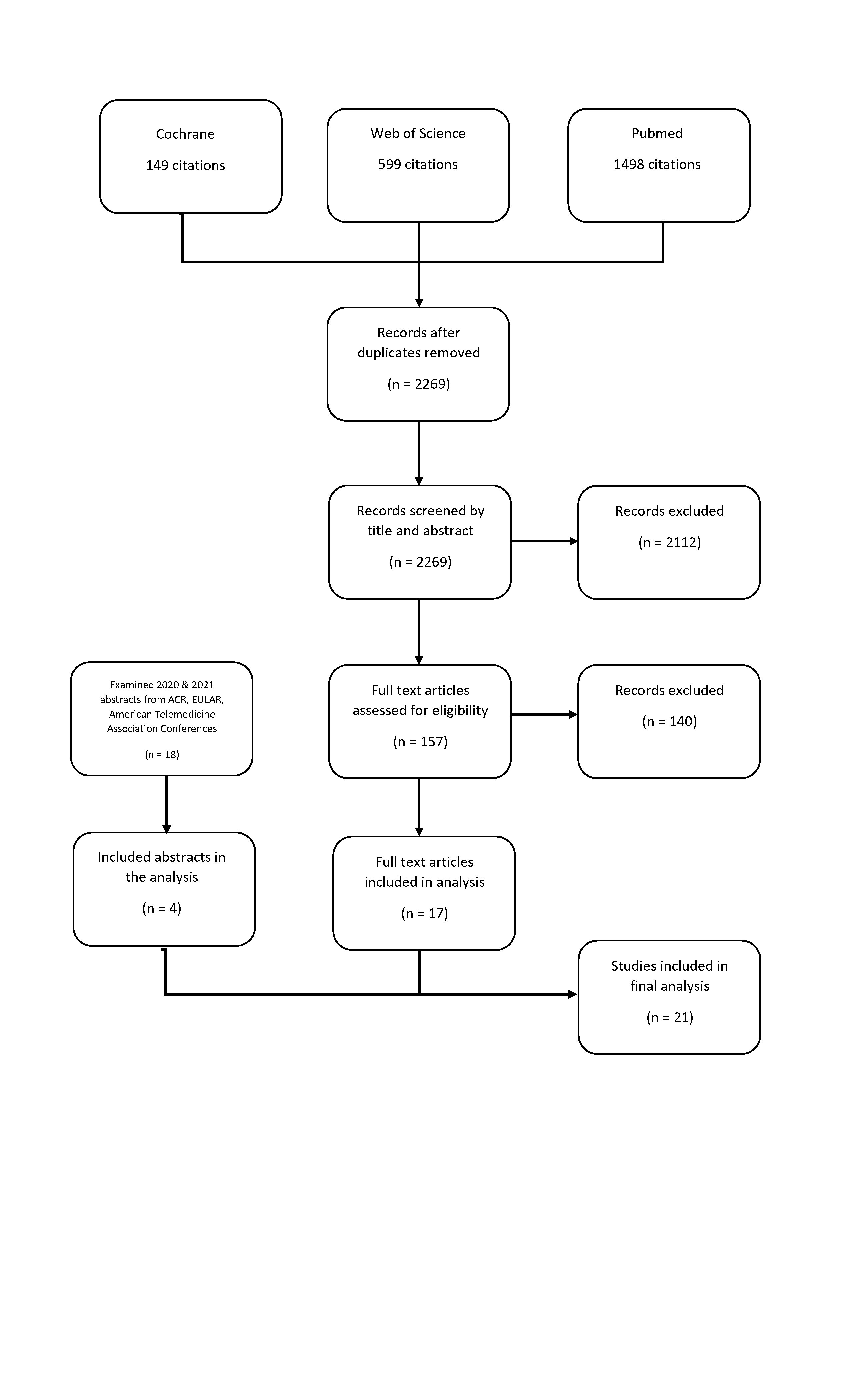 Figure 2: Process Used to Retrieve Relevant Articles & Abstracts
Figure 2: Process Used to Retrieve Relevant Articles & Abstracts
Disclosures: A. Peck, None; R. Grainger, AbbVie/Abbott, Janssen, Novartis, Pfizer, Cornerstones; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; J. Cush, None; N. Soares, None; N. Davuluri, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; S. Sodhi, None; D. Grauer, None; N. Fortune, None; S. Venkatachalam, None; D. Kirby, None; J. Alper, None; K. Gavigan, None; L. Stradford, Global Healthy Living Foundation; D. Curtis, Global Healthy Living Foundation; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen.
Background/Purpose: Home based telehealth (HBT) visits, where a patient is located at home with a remote provider, became common during the COVID-19 pandemic. Patients now request HBT, even though in-person visits are possible. We conducted a systematic literature search with narrative synthesis on the use of HBT in rheumatology with the aim of informing the clinical practice of rheumatology using HBT.
Methods: We searched Cochrane, PubMed, and Web of Science databases using a pre-defined search strategy (Figure 1). A total of 2269 articles were retrieved and then subjected to further review (Figure 2). Two independent reviewers assessed whether the articles specifically addressed our objectives of determining HBT suitability, choosing telehealth-appropriate disease outcome measures, and identifying instruments suitable for measuring patient acceptance of HBT. Data was then extracted for a narrative summary and gap analysis.
Results: Seventeen studies and four abstracts met inclusion criteria and were included in the synthesis. Study heterogeneity precluded a meta-analysis. The key findings were as follows: Two studies support the use of pre-visit triage for new patients with inflammatory back pain and for early inflammatory arthritis by using telephone survey tools and enrolling Spondyloarthropathy patients for inflammatory back pain and rheumatologist-diagnosed inflammatory arthritis patients as control groups, respectively. Two survey studies reported providers had good acceptance of HBT for stable patients compared to more active patients or more complex presentations. Four studies reported successful use of remotely collected disease activity measures and PROs and reported concordance between PROs (RAID and RAPID3) with DAS-28 in low disease activity but not higher disease activity states. Another study supported the use of protocolized serum uric acid management in gout via telehealth visits. Two studies examined reported that telehealth satisfaction was generally high but was lower with increasing patient age.
Conclusion: A systematic literature search for articles addressing HBT in rheumatology provides useful, albeit limited, data as to how HBT can safely and effectively complement in-person care for certain rheumatologic conditions, particularly inflammatory arthritis, and gout. The data also provides some guidance on the appropriate selection of patients for telehealth. Further, the data highlights key gaps that future research should address such as creating strategies to identify patients whose health needs can be adequately addressed by HBT and optimizing methods to collect PROs and disease activity measures during HBT.
 Figure 1: Search Strategy used to Retrieve Telehealth Articles
Figure 1: Search Strategy used to Retrieve Telehealth Articles Figure 2: Process Used to Retrieve Relevant Articles & Abstracts
Figure 2: Process Used to Retrieve Relevant Articles & AbstractsDisclosures: A. Peck, None; R. Grainger, AbbVie/Abbott, Janssen, Novartis, Pfizer, Cornerstones; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; J. Cush, None; N. Soares, None; N. Davuluri, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; S. Sodhi, None; D. Grauer, None; N. Fortune, None; S. Venkatachalam, None; D. Kirby, None; J. Alper, None; K. Gavigan, None; L. Stradford, Global Healthy Living Foundation; D. Curtis, Global Healthy Living Foundation; S. Venuturupalli, Horizon Pharma USA, Inc., Kezar Life Sciences, Inc., Mallinckrodt, Inc., Navidea Biopharmaceuticals, Inc., Pfizer (Current), Bristol-Myers Squibb(BMS), Argenx, Janssen.

