Back
Poster Session B
Vasculitis
Session: (1071–1093) Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
1091: IgG Antibody Subclass Glycan Modifications in Patients with ANCA-Associated Vasculitis in Active vs Remission States – a Proof-of-Concept Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- ZW
Zachary Wallace, MD, MSc
Massachusetts General Hospital
Newton, MA, United States
Abstract Poster Presenter(s)
Zachary Wallace1, Tina Motazedi1, Michelle Conroy1, Claire Cook1, John Stone2, Hyon Choi3 and Robert Anthony1, 1Massachusetts General Hospital, Boston, MA, 2Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA, 3MASSACHUSETTS GENERAL HOSPITAL, Lexington, MA
Background/Purpose: The Fc portion of IgG tends to be hypogalactosylated among patients with ANCA-associated vasculitis (AAV) compared with healthy controls1,2,3. Glycan modifications may affect antibody effector functions and therefore serve as a marker of disease activity2. A prior study found that glycosylation patterns differed in PR3- but not MPO-ANCA+ AAV patients with active disease vs remission3. In particular, a higher proportion of total IgG had agalactosylation during active disease vs remission in PR3-ANCA+ AAV. To date, few studies have evaluated glycosylation patterns in MPO-ANCA+ AAV, variations in glycosylation patterns of IgG subclasses in AAV, or these differences in the context of B cell depletion or persistent ANCA positivity.
Methods: We identified patients from the Mass General Brigham (MGB) AAV cohort who had serum samples available from the MGB Biobank or in our prospective MGB AAV cohort. Of these, we included samples from three patients collected at the time of their initial AAV presentation but prior to any treatment and from three patients collected while they were on B cell depletion and their disease was in clinical remission. IgG subclass specific glycosylation was determined by glycopeptide mass spectrometry. We assessed the proportion of IgG1, IgG2, and IgG3, respectively, that was agalactosylated, galactosylated, sialylated, or bisected.
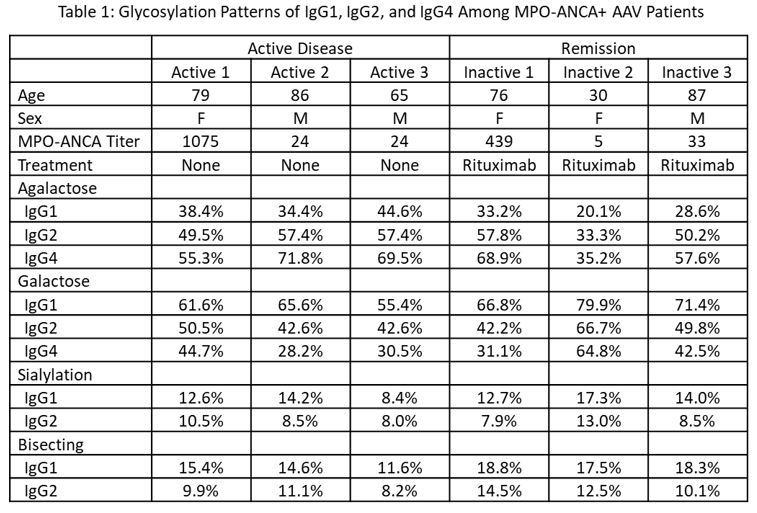
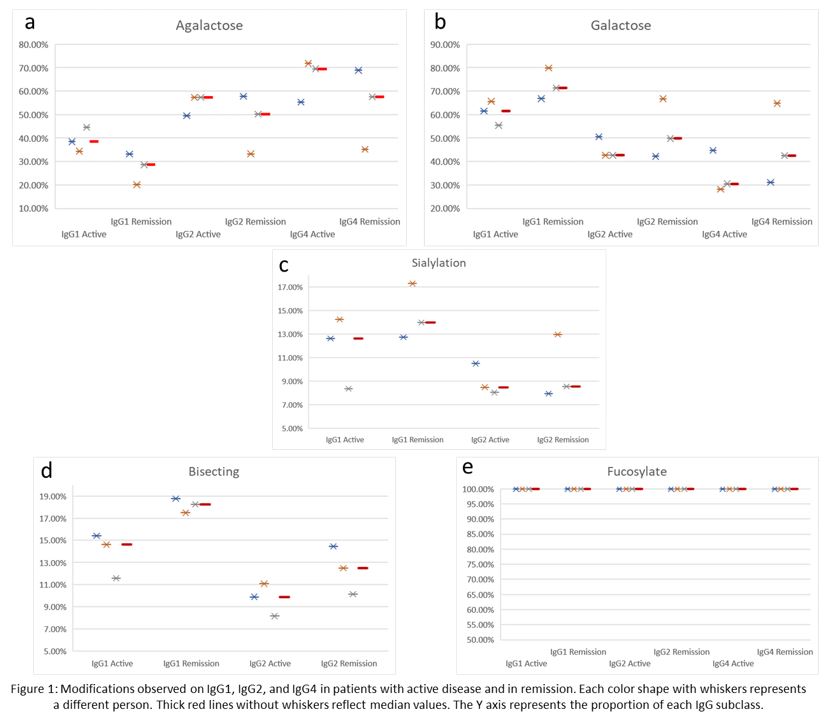
Results: We included six patients, three with newly diagnosed AAV prior to any treatment and three in remission on rituximab for maintenance of remission. All six patients were MPO-ANCA+ by ELISA at the time of sample collection with titers ranging from 5 to 1,075 units (Table 1). Patients with active disease tended to have higher proportions of IgG1, IgG2, and IgG4 that was agalactosylated and lower proportions that were galactosylated when compared with patients in remission (Figures 1a and 1b). Sialylation patterns were similar on IgG1 and IgG2 in active disease vs remission; sialylation was not detected on IgG4 (Figure 1c). Bisecting species were detected on a lower proportion of IgG1 and IgG2 in active disease than in remission (Figure 1d). There was no difference in the proportion of fucosylated IgG1, IgG2, and IgG4 in active disease and remission (Figure 1e).
Conclusion: This proof-of-concept study suggests strong trends in glycosylation pattern differences of IgG1, IgG2, and IgG4 when comparing MPO-ANCA+ AAV patients with active disease vs those in remission, despite having persistently elevated ANCA titers. In particular, active MPO-ANCA+ AAV was characterized by a greater proportion of agalactosylated antibody, similar to what has been described in PR3-ANCA+ AAV. These results, in combination with prior studies, suggest that differences in glycosylation may be a promising avenue for future investigation in both PR3- and MPO-ANCA+ AAV where reliable biomarkers of disease activity are urgently needed. These data call for a full-scale investigation to assess MPO- and PR3-ANCA and leverage longitudinal serial samples to further assess glycosylation patterns as a biomarker.
1Clin Exp Immunol 2002;129:183; 2JASN 2010;21:1103; 3PloS One 2019;14:e0213215
Disclosures: Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; T. Motazedi, None; M. Conroy, None; C. Cook, None; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas; H. Choi, Horizon, Allena, LG, Protalix; R. Anthony, None.
Background/Purpose: The Fc portion of IgG tends to be hypogalactosylated among patients with ANCA-associated vasculitis (AAV) compared with healthy controls1,2,3. Glycan modifications may affect antibody effector functions and therefore serve as a marker of disease activity2. A prior study found that glycosylation patterns differed in PR3- but not MPO-ANCA+ AAV patients with active disease vs remission3. In particular, a higher proportion of total IgG had agalactosylation during active disease vs remission in PR3-ANCA+ AAV. To date, few studies have evaluated glycosylation patterns in MPO-ANCA+ AAV, variations in glycosylation patterns of IgG subclasses in AAV, or these differences in the context of B cell depletion or persistent ANCA positivity.
Methods: We identified patients from the Mass General Brigham (MGB) AAV cohort who had serum samples available from the MGB Biobank or in our prospective MGB AAV cohort. Of these, we included samples from three patients collected at the time of their initial AAV presentation but prior to any treatment and from three patients collected while they were on B cell depletion and their disease was in clinical remission. IgG subclass specific glycosylation was determined by glycopeptide mass spectrometry. We assessed the proportion of IgG1, IgG2, and IgG3, respectively, that was agalactosylated, galactosylated, sialylated, or bisected.
Results: We included six patients, three with newly diagnosed AAV prior to any treatment and three in remission on rituximab for maintenance of remission. All six patients were MPO-ANCA+ by ELISA at the time of sample collection with titers ranging from 5 to 1,075 units (Table 1). Patients with active disease tended to have higher proportions of IgG1, IgG2, and IgG4 that was agalactosylated and lower proportions that were galactosylated when compared with patients in remission (Figures 1a and 1b). Sialylation patterns were similar on IgG1 and IgG2 in active disease vs remission; sialylation was not detected on IgG4 (Figure 1c). Bisecting species were detected on a lower proportion of IgG1 and IgG2 in active disease than in remission (Figure 1d). There was no difference in the proportion of fucosylated IgG1, IgG2, and IgG4 in active disease and remission (Figure 1e).
Conclusion: This proof-of-concept study suggests strong trends in glycosylation pattern differences of IgG1, IgG2, and IgG4 when comparing MPO-ANCA+ AAV patients with active disease vs those in remission, despite having persistently elevated ANCA titers. In particular, active MPO-ANCA+ AAV was characterized by a greater proportion of agalactosylated antibody, similar to what has been described in PR3-ANCA+ AAV. These results, in combination with prior studies, suggest that differences in glycosylation may be a promising avenue for future investigation in both PR3- and MPO-ANCA+ AAV where reliable biomarkers of disease activity are urgently needed. These data call for a full-scale investigation to assess MPO- and PR3-ANCA and leverage longitudinal serial samples to further assess glycosylation patterns as a biomarker.
1Clin Exp Immunol 2002;129:183; 2JASN 2010;21:1103; 3PloS One 2019;14:e0213215


Disclosures: Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; T. Motazedi, None; M. Conroy, None; C. Cook, None; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas; H. Choi, Horizon, Allena, LG, Protalix; R. Anthony, None.

